To assess the epidemiology and outcome at discharge of cancer patients requiring admission to the Intensive Care Unit (ICU).
DesignA descriptive observational study was made of data from the ENVIN-HELICS registry, combined with specifically compiled variables. Comparisons were made between patients with and without neoplastic disease, and groups of cancer patients with a poorer outcome were identified.
SettingIntensive Care Units participating in ENVIN-HELICS 2018, with voluntary participation in the oncological registry.
PatientsSubjects admitted during over 24 h and diagnosed with cancer in the last 5 years.
Primary endpointsThe general epidemiological endpoints of the ENVIN-HELICS registry and cancer-related variables.
ResultsOf the 92 ICUs with full data, a total of 11,796 patients were selected, of which 1786 (15.1%) were cancer patients. The proportion of cancer patients per Unit proved highly variable (1%–48%). In-ICU mortality was higher among the cancer patients than in the non-oncological subjects (12.3% versus 8.9%; p < .001). Elective postoperative (46.7%) or emergency admission (15.3%) predominated in the cancer patients. Patients with medical disease were in more serious condition, with longer stay and greater mortality (27.5%). The patients admitted to the ICU due to nonsurgical disease related to cancer exhibited the highest mortality rate (31.4%).
ConclusionsGreat variability was recorded in the percentage of cancer patients in the different ICUs. A total of 46.7% of the patients were admitted after undergoing scheduled surgery. The highest mortality rate corresponded to patients with medical disease (27.5%), and to those admitted due to cancer-related complications (31.4%).
Conocer la epidemiología y evolución al alta de los pacientes oncológicos que precisan ingreso en UCI.
DiseñoEstudio descriptivo observacional de datos del registro ENVIN-HELICS combinado con variables registradas específicamente. Se comparan pacientes con y sin neoplasia. Se identifican grupos de pacientes neoplásicos con peor evolución.
ÁmbitoUCI participantes en ENVIN-HELICS del año 2018 con participación voluntaria en el registro oncológico.
PacientesIngresados más de 24 horas. Entre estos aquellos diagnosticados de neoplasia en los últimos 5 años.
Variables principalesLas generales epidemiológicas del registro ENVIN-HELICS y variables relacionadas con la neoplasia.
ResultadosEn las 92 UCI con datos completos se seleccionaron 11.796 pacientes, de los que 1.786 (15,1%) son pacientes con neoplasia. La proporción de pacientes con cáncer por unidad fue muy variable (rango: 1–48%). La mortalidad en UCI de los pacientes oncológicos fue superior a los no oncológicos (12,3% versus 8,9%; p < 0,001). En pacientes oncológicos predominaron los ingresados en el postoperatorio programado (46,7%) o urgente (15,3%). Los pacientes con proceso patológico médico fueron más graves, con mayor estancia y mortalidad (27,5%). Aquellos ingresados en UCI por enfermedad no quirúrgica relacionada con el cáncer tuvieron la mortalidad más alta (31,4%).
ConclusiónExiste una gran variabilidad en el porcentaje de pacientes oncológicos en las diferentes UCI. El 46,7% de los pacientes ingresa tras someterse a cirugía programada. La mayor mortalidad corresponde a pacientes con enfermedad médica (27,5%) y a los ingresados por complicaciones relacionadas con el cáncer (31,4%).
The incidence of cancer is increasing, and the disease is responsible for 25% of overall mortality in Spain.1 Improvements in active treatments and supportive care collaborate in reducing mortality associated to cancer. In this regard, patients with neoplasms represent a population that is currently susceptible to admission to the Intensive Care Unit (ICU) as part of cancer treatment, for the treatment of intercurrent medical and surgical processes, or as a key tool for the management of toxicity caused by the different oncological therapies.2
Such improvement in survival makes it necessary to reconsider the role of intensive care medicine in this scenario. Different scientific associations are interested in understanding this role; as a result, contacts have begun to be established among scientific groups interested in this subject.3 In Spain, a collaboration agreement was signed between the Spanish Society of Intensive and Critical Care Medicine and Coronary Units (Sociedad Española de Medicina Intensiva, Crítica y Unidades Coronarias [SEMICYUC]) and the Spanish Society of Medical Oncology (Sociedad Española de Oncología Médica [SEOM]), contemplating the creation of a registry to generate information on the epidemiology and factors related to mortality among oncological patients requiring admission to the ICU.4
In recent years, studies have been made of survival in the ICU among different highly selected groups of oncological patients5–9 (involving mainly hematological malignancies), and recommendations have been made in relation to the different therapeutic strategies.2,10 Studies have also been made on the evolution of cancer patients subjected to certain therapeutic procedures inherent to intensive care, such as mechanical ventilation,11,12 high-flow oxygen therapy,13 renal replacement techniques14 or even extracorporeal oxygenation.15
However, few general epidemiological data are available on the impact of cancer patient admission to the ICU. In multicenter studies, the proportion of patients with cancer admitted to the ICU ranges between 13%–20%.16–21 Nevertheless, this population is extremely heterogeneous — a fact that may have an impact upon the care they require. It is clear that a close multiprofessional and multidisciplinary approach is the only possible way to improve the prognosis of critical patients with cancer.4,22–26
The present study was carried out to know the epidemiology, reasons for admission, the therapeutic resources used, and mortality among patients with malignant disease according to the causes giving rise to their admission to the ICU.
Patients and methodsAn observational study was carried out based on the ENVIN registry (which we refer to as the “ENVIN database”). All the patients admitted between 1 April and 30 June 2018 were included. Expansion of the data on the cancer patients was made by combining a new database referred to as the “ONCOENVIN database”. Three common variables (age, date of admission and gender) served to generate a common identifier allowing data linkage between the two databases.
Selection of patients in the ENVIN databaseThe ENVIN registry is a period prevalence and multicenter (Spanish national), voluntary participation observational registry. It was developed in 1994 by the Infectious Diseases and Sepsis Study Group of the SEMICYUC. The purpose of the registry was to record the frequency and etiology of the infections associated to devices used in the ICU. It likewise records consumption of all the antimicrobials used during the study period, as well as the prevalence of multiresistant pathogens related to colonization and infection in the ICU.
Since the year 1994, there has been an increase in the voluntary participation of different ICUs, reaching a total of 219 Units pertaining to 185 hospitals in 2018. Data input is made using a software application available at: http://hws.vhebron.net/envin-helics/. The ENVIN registry has been approved by different local and regional Clinical Research Ethics Committees (CRECs). No express permission from the patients is required for the use of their data, since the registry is recognized as being an instrument of interest to the Spanish National Healthcare System (year 2014).
The registry records the presence of neoplastic disease (both hematological and solid organ tumors) when the latter was diagnosed up to 5 years before patient admission to the ICU, or during admission itself. The information of the ONCOENVIN database could only be completed in the case of patients in which this circumstance was confirmed.
Other recorded data referred to the size of the Units, the methodology involved in the use of devices and the development of infections have been previously published27–29 and are provided as electronic supplementary information.
Selection of patients in the ONCOENVIN databaseIn the case of those patients with a confirmed history of cancer (as a prior diagnosis or diagnosed during hospital admission), the variables related to the disease were entered voluntarily. We first considered whether admission to the ICU was due to causes related to the neoplasm or not. In each case we took into account whether the reason for admission was the providing of immediate postoperative care in the context of surgery related or not related to the malignancy. With regard to the non-surgical patients, grouping was made of the subjects admitted due to medical complications related with the neoplasm, including the following reasons: respiratory failure, sepsis/septic shock, coma, metabolic disorders, renal failure, hemorrhagic shock, the administration of chemotherapy, or other medical causes related to the neoplasm.
On the other hand, a distinction was made between hematological malignancies and solid organ tumors. The former in turn were classified into lymphomas, leukemias and other hematological malignancies. The anatomical location of the solid tumors was recorded. Likewise, we studied the year in which the neoplasm had been diagnosed, excluding all cases diagnosed before the year 2013.
The antineoplastic treatment which the patients were receiving at the time of admission to the ICU was considered. This treatment was classified as neoadjuvant therapy (therapy prior to main treatment, generally – but not always – involving surgery), adjuvant therapy (complementary treatment following the main treatment), treatment with radical intent, and treatment with first or successive lines against metastatic disease. Lastly, symptomatic treatment was defined as corresponding to those patients who were receiving no active treatment or who were only receiving supportive or purely symptomatic therapy (e.g., for pain).
On the other hand, we also recorded those treatments specifically targeted to hematological malignancies such as allogenic bone marrow transplantation, autologous bone marrow transplantation and chemotherapy for acute leukemia (whether induction, consolidation or maintenance therapy). Finally, the category “other treatments” was represented by those treatments that did not meet the above definitions, including hormone therapy, chemotherapy with abdominal intracavitary hyperthermia, as well as palliative therapies and the absence of any specific anticancer treatment at the time of patient admission to the ICU.
During patient stay in the ICU, we recorded the development of neutropenia (<500 neutrophils per mm3) not present at the time of admission to the ICU; the administration of chemotherapy during admission to the ICU; tumor lysis syndrome according to the criteria of Cairo and Bishop30; the limitation of life support measures (referred to both withdrawal and the non-initiation of a treatment); and the diagnosis of pulmonary aspergillosis (consistent clinical data plus serum or bronchoalveolar lavage [BAL] galactomannan, or isolation of Aspergillus spp. in respiratory sample culture).
Statistical analysisBoth databases, located in different servers (pertaining to Hospital Vall d’Hebron and the SEMICYUC, respectively), were pooled using the common identifier that did not allow identification of the patient. Qualitative variables were reported as a percentage, and quantitative variables as the mean and standard deviation (SD) or as the median and interquartile range (p25–p75) in the absence of normal data distribution. Bivariate analysis was based on the chi-square test for qualitative variables and the Mann–Whitney U-test (2 samples) or Kruskal–Wallis test (>2 samples) for quantitative variables. No analysis of mortality-related factors was made, but mortality in the ICU related to time was studied based on Kaplan–Meier curves applied to the general population, with differentiation according to the reason for admission and its relation to malignant disease. The outcome discharge/death was censored at 60 days of stay in the ICU. Statistical significance was considered for p < 0.05 in all cases. The SPSS version 23.0 statistical package was used throughout.
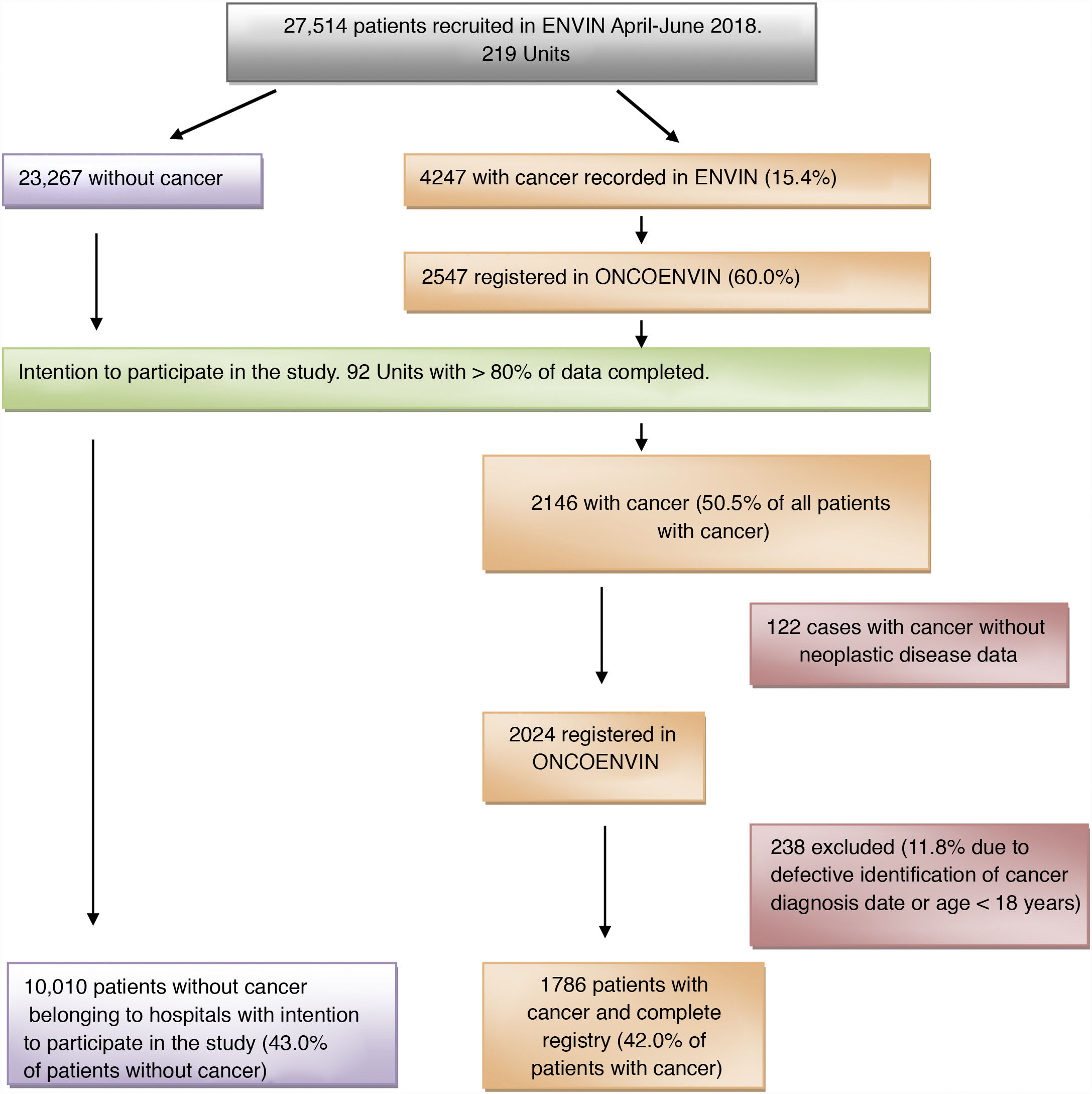
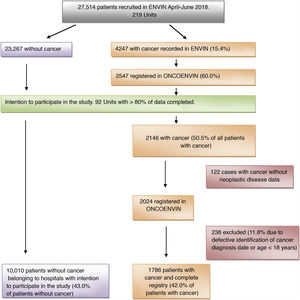
ResultsIn the year 2018, the full ENVIN period (April–June) had recorded 27,514 patients admitted to some of the 219 Units that participated that year in the registry. A total of 4247 patients (15.4%) had a history of cancer. Of these, 60% (2547 patients) contributed full data of the ONCOENVIN database. Since participation was voluntary and open, we considered that those Units which in ONCOENVIN had entered fewer than 80% of the cases that had been declared in ENVIN should be excluded. A total of 92 Units completed over 80% of the cases, and their data were therefore considered valid for the epidemiological study. One hundred and twenty-two patients (5.7%) of these Units intending to participate did not have the oncological information. On the other hand, 238 patients were excluded due to defective identification of the date of diagnosis of the malignancy or an age of under 18 years. Finally, we analyzed a total of 1786 patients with cancer disease and a complete registry (42% of the patients with cancer) versus 10,010 patients without cancer belonging to the Units intending to participate (43% of the patients without cancer). Thus, in this selected population, the cancer patients represented 15.1% of the total of patients. Fig. 1 shows the patient screening process for the epidemiological study.
The percentage of cancer patients with respect to the total admissions in the 92 selected Units was highly variable, with a median of 17 patients (p25–p75: 10–24.5) per Unit, but with a proportion of between 1%–48% of cancer patients with respect to the total per Unit. This variation reflects the diversity of the characteristics of the participating ICUs, which included coronary Units (obviously with a very low percentage of cancer patients) and small postsurgery Units with very high percentages of cancer patients. Overall, 87% of the Units were considered to be polyvalent, while 3.3% were exclusively postsurgery Units, and the same proportion consisted of trauma Units. The geographical distribution was uniform, with representation of all the Spanish regions (Autonomous Communities), and a total of 38 provinces.
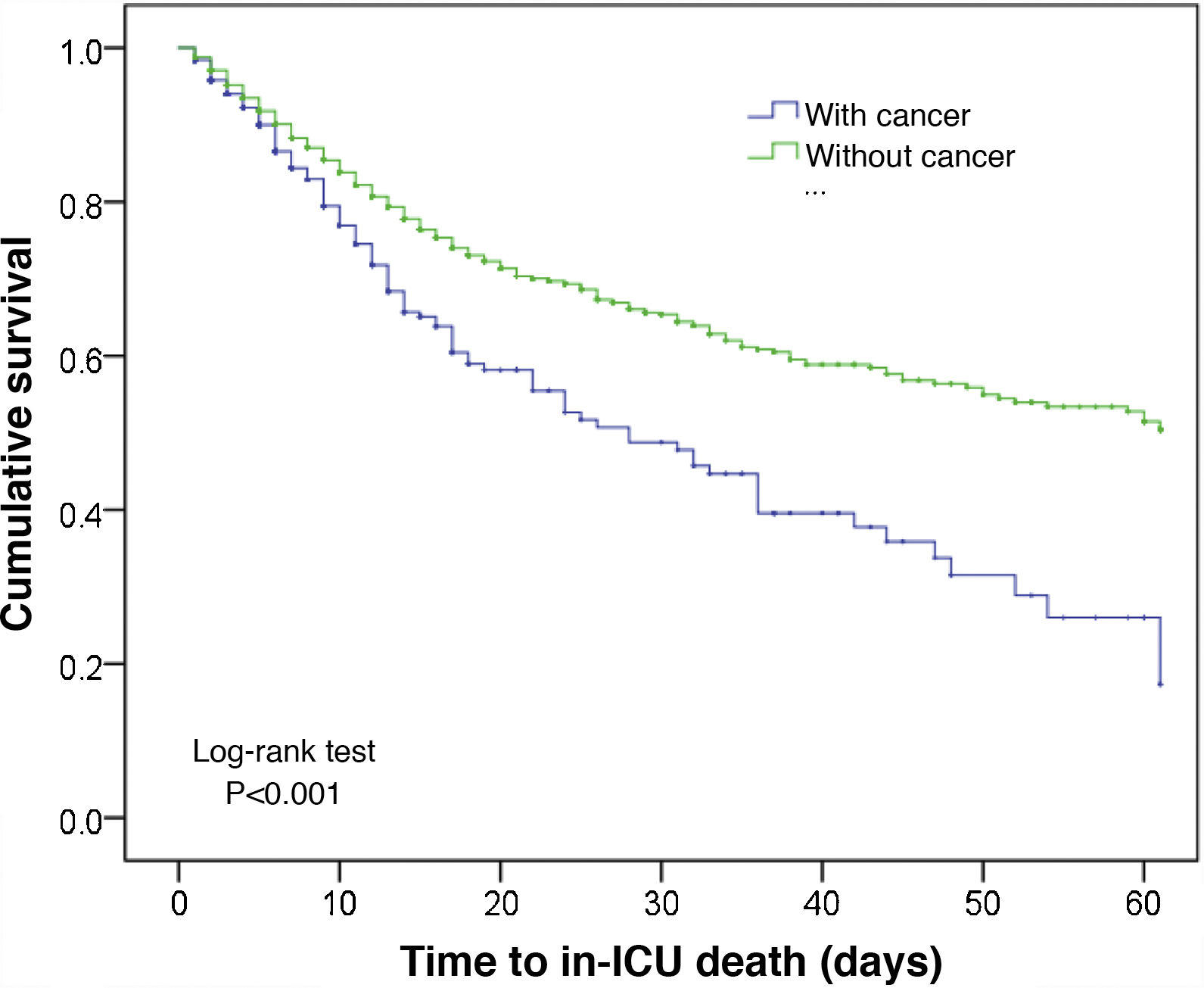
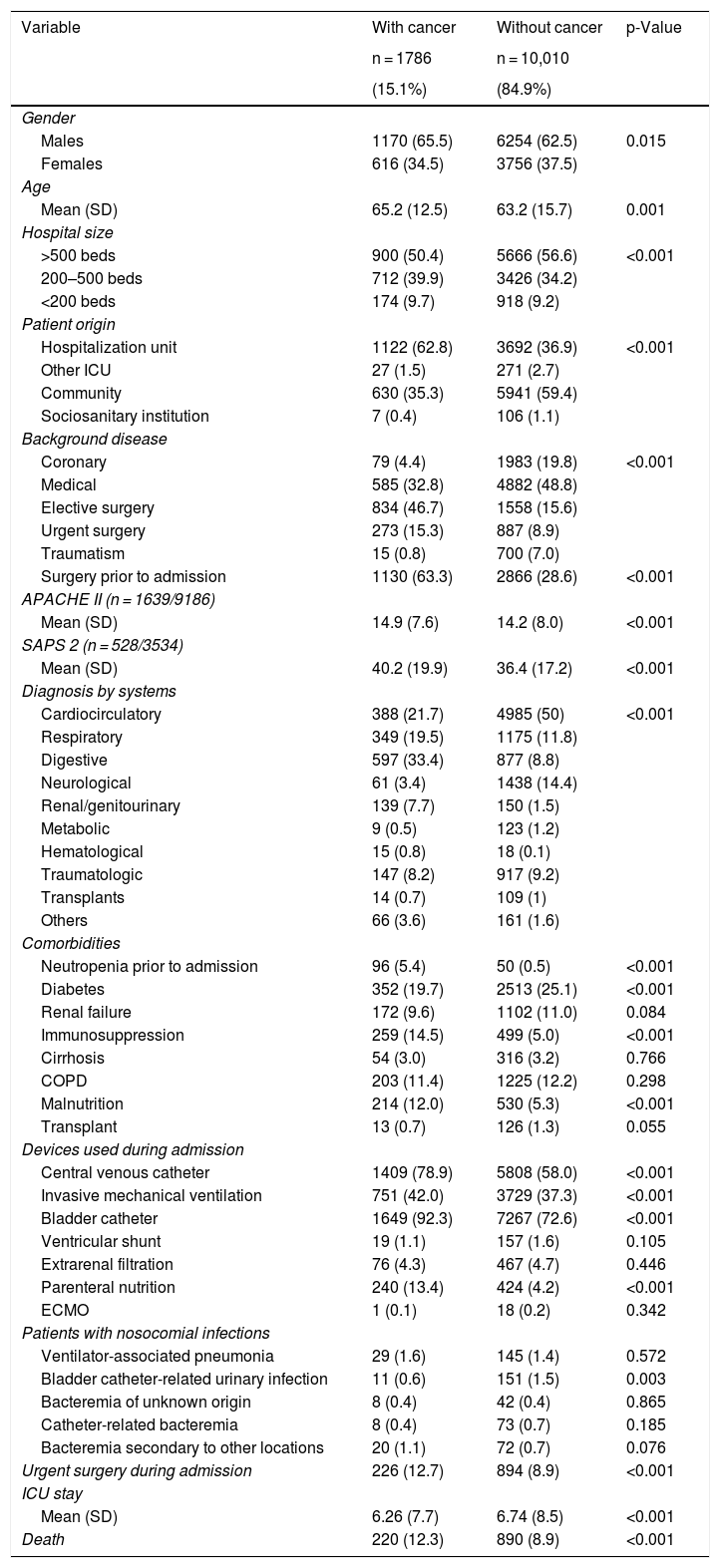
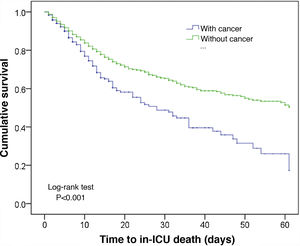
In comparison with the patients without cancer, those subjects with a recent history of cancer were generally older, came from hospital wards, with a predominance of surgical antecedents, greater severity upon admission, a shorter stay, and greater in-ICU mortality (12.3% versus 8.5%; p < 0.001). These data are shown in Table 1. The Kaplan–Meier curves reflecting the survival time differences are shown in Fig. 2 (log-rank test, p < 0.001).
Comparison of patients with and without cancer admitted to the selected Units.
| Variable | With cancer | Without cancer | p-Value |
|---|---|---|---|
| n = 1786 | n = 10,010 | ||
| (15.1%) | (84.9%) | ||
| Gender | |||
| Males | 1170 (65.5) | 6254 (62.5) | 0.015 |
| Females | 616 (34.5) | 3756 (37.5) | |
| Age | |||
| Mean (SD) | 65.2 (12.5) | 63.2 (15.7) | 0.001 |
| Hospital size | |||
| >500 beds | 900 (50.4) | 5666 (56.6) | <0.001 |
| 200–500 beds | 712 (39.9) | 3426 (34.2) | |
| <200 beds | 174 (9.7) | 918 (9.2) | |
| Patient origin | |||
| Hospitalization unit | 1122 (62.8) | 3692 (36.9) | <0.001 |
| Other ICU | 27 (1.5) | 271 (2.7) | |
| Community | 630 (35.3) | 5941 (59.4) | |
| Sociosanitary institution | 7 (0.4) | 106 (1.1) | |
| Background disease | |||
| Coronary | 79 (4.4) | 1983 (19.8) | <0.001 |
| Medical | 585 (32.8) | 4882 (48.8) | |
| Elective surgery | 834 (46.7) | 1558 (15.6) | |
| Urgent surgery | 273 (15.3) | 887 (8.9) | |
| Traumatism | 15 (0.8) | 700 (7.0) | |
| Surgery prior to admission | 1130 (63.3) | 2866 (28.6) | <0.001 |
| APACHE II (n = 1639/9186) | |||
| Mean (SD) | 14.9 (7.6) | 14.2 (8.0) | <0.001 |
| SAPS 2 (n = 528/3534) | |||
| Mean (SD) | 40.2 (19.9) | 36.4 (17.2) | <0.001 |
| Diagnosis by systems | |||
| Cardiocirculatory | 388 (21.7) | 4985 (50) | <0.001 |
| Respiratory | 349 (19.5) | 1175 (11.8) | |
| Digestive | 597 (33.4) | 877 (8.8) | |
| Neurological | 61 (3.4) | 1438 (14.4) | |
| Renal/genitourinary | 139 (7.7) | 150 (1.5) | |
| Metabolic | 9 (0.5) | 123 (1.2) | |
| Hematological | 15 (0.8) | 18 (0.1) | |
| Traumatologic | 147 (8.2) | 917 (9.2) | |
| Transplants | 14 (0.7) | 109 (1) | |
| Others | 66 (3.6) | 161 (1.6) | |
| Comorbidities | |||
| Neutropenia prior to admission | 96 (5.4) | 50 (0.5) | <0.001 |
| Diabetes | 352 (19.7) | 2513 (25.1) | <0.001 |
| Renal failure | 172 (9.6) | 1102 (11.0) | 0.084 |
| Immunosuppression | 259 (14.5) | 499 (5.0) | <0.001 |
| Cirrhosis | 54 (3.0) | 316 (3.2) | 0.766 |
| COPD | 203 (11.4) | 1225 (12.2) | 0.298 |
| Malnutrition | 214 (12.0) | 530 (5.3) | <0.001 |
| Transplant | 13 (0.7) | 126 (1.3) | 0.055 |
| Devices used during admission | |||
| Central venous catheter | 1409 (78.9) | 5808 (58.0) | <0.001 |
| Invasive mechanical ventilation | 751 (42.0) | 3729 (37.3) | <0.001 |
| Bladder catheter | 1649 (92.3) | 7267 (72.6) | <0.001 |
| Ventricular shunt | 19 (1.1) | 157 (1.6) | 0.105 |
| Extrarenal filtration | 76 (4.3) | 467 (4.7) | 0.446 |
| Parenteral nutrition | 240 (13.4) | 424 (4.2) | <0.001 |
| ECMO | 1 (0.1) | 18 (0.2) | 0.342 |
| Patients with nosocomial infections | |||
| Ventilator-associated pneumonia | 29 (1.6) | 145 (1.4) | 0.572 |
| Bladder catheter-related urinary infection | 11 (0.6) | 151 (1.5) | 0.003 |
| Bacteremia of unknown origin | 8 (0.4) | 42 (0.4) | 0.865 |
| Catheter-related bacteremia | 8 (0.4) | 73 (0.7) | 0.185 |
| Bacteremia secondary to other locations | 20 (1.1) | 72 (0.7) | 0.076 |
| Urgent surgery during admission | 226 (12.7) | 894 (8.9) | <0.001 |
| ICU stay | |||
| Mean (SD) | 6.26 (7.7) | 6.74 (8.5) | <0.001 |
| Death | 220 (12.3) | 890 (8.9) | <0.001 |
APACHE: Acute Physiology, Age and Chronic Health Evaluation; SD: standard deviation; ECMO: extracorporeal membrane oxygenation; COPD: chronic obstructive pulmonary disease; SAPS: Simplified Acute Physiology Score.
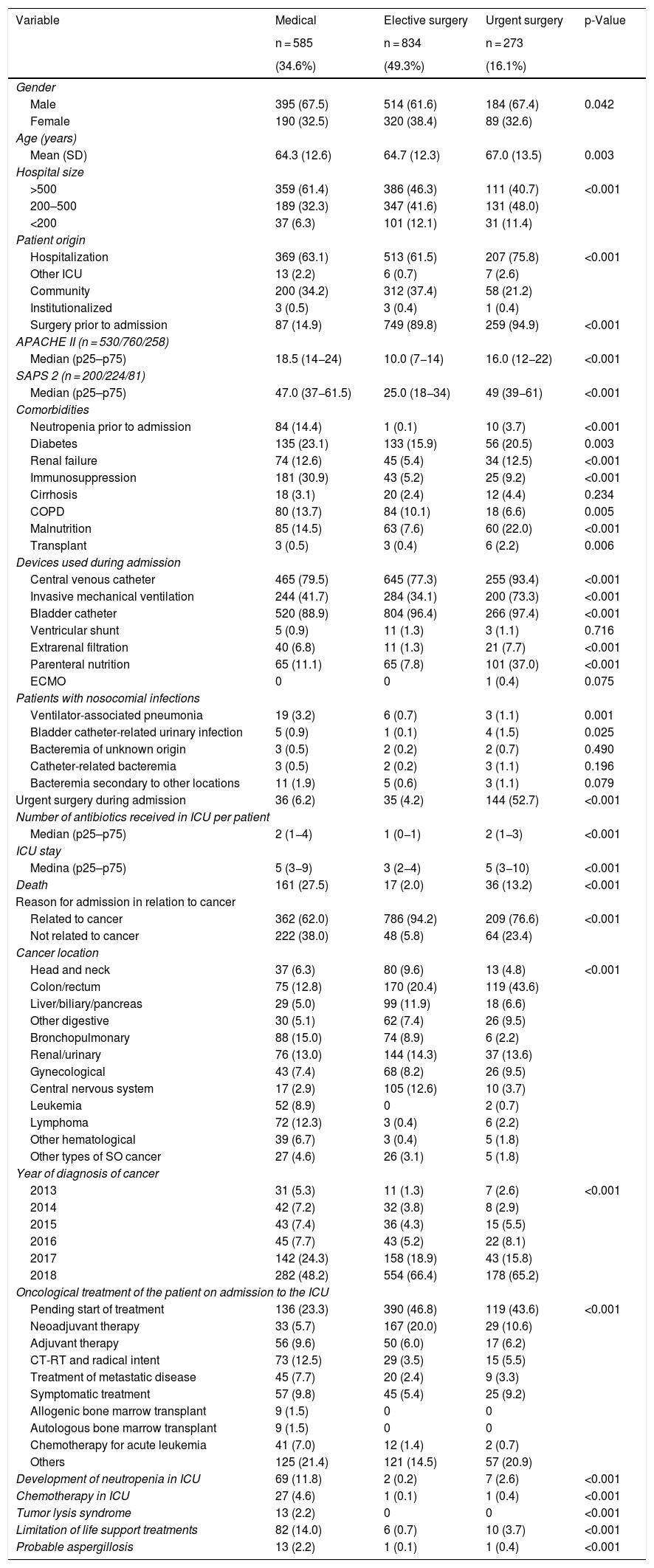
In order to assess the type of patient admitted to the ICU, and taking into account the prevalence of surgical cases among the cancer patients, a distribution was established according to whether the patients suffered a medical disease condition (n = 585) or had been admitted after elective surgery (n = 834) or after urgent surgery (n = 273). Coronary and trauma cases were excluded because of their scant representation (5.2% overall) and lack of influence in relation to the objective of our study. In general, the patients with a background medical disease condition were more seriously ill, with a greater percentage of comorbidities and of infectious complications, and with significant higher mortality (27%) than the patients subjected to elective surgery (2%) or urgent surgery (13,2%). The rest of the differential characteristics are shown in Table 2. Obviously, the hematological patients were more often admitted due to medical causes (89.6%) than to causes related to elective surgery (3.3%) or urgent surgery (7.1%). In contrast, the patients with solid organ tumors were more often admitted due to overall surgical causes (72.1%) than to medical causes (27.9%).
Comparative analysis between patients with cancer according to background disease causing admission to the ICU. Coronary and trauma cases are excluded.
| Variable | Medical | Elective surgery | Urgent surgery | p-Value |
|---|---|---|---|---|
| n = 585 | n = 834 | n = 273 | ||
| (34.6%) | (49.3%) | (16.1%) | ||
| Gender | ||||
| Male | 395 (67.5) | 514 (61.6) | 184 (67.4) | 0.042 |
| Female | 190 (32.5) | 320 (38.4) | 89 (32.6) | |
| Age (years) | ||||
| Mean (SD) | 64.3 (12.6) | 64.7 (12.3) | 67.0 (13.5) | 0.003 |
| Hospital size | ||||
| >500 | 359 (61.4) | 386 (46.3) | 111 (40.7) | <0.001 |
| 200–500 | 189 (32.3) | 347 (41.6) | 131 (48.0) | |
| <200 | 37 (6.3) | 101 (12.1) | 31 (11.4) | |
| Patient origin | ||||
| Hospitalization | 369 (63.1) | 513 (61.5) | 207 (75.8) | <0.001 |
| Other ICU | 13 (2.2) | 6 (0.7) | 7 (2.6) | |
| Community | 200 (34.2) | 312 (37.4) | 58 (21.2) | |
| Institutionalized | 3 (0.5) | 3 (0.4) | 1 (0.4) | |
| Surgery prior to admission | 87 (14.9) | 749 (89.8) | 259 (94.9) | <0.001 |
| APACHE II (n = 530/760/258) | ||||
| Median (p25–p75) | 18.5 (14−24) | 10.0 (7−14) | 16.0 (12−22) | <0.001 |
| SAPS 2 (n = 200/224/81) | ||||
| Median (p25–p75) | 47.0 (37−61.5) | 25.0 (18−34) | 49 (39−61) | <0.001 |
| Comorbidities | ||||
| Neutropenia prior to admission | 84 (14.4) | 1 (0.1) | 10 (3.7) | <0.001 |
| Diabetes | 135 (23.1) | 133 (15.9) | 56 (20.5) | 0.003 |
| Renal failure | 74 (12.6) | 45 (5.4) | 34 (12.5) | <0.001 |
| Immunosuppression | 181 (30.9) | 43 (5.2) | 25 (9.2) | <0.001 |
| Cirrhosis | 18 (3.1) | 20 (2.4) | 12 (4.4) | 0.234 |
| COPD | 80 (13.7) | 84 (10.1) | 18 (6.6) | 0.005 |
| Malnutrition | 85 (14.5) | 63 (7.6) | 60 (22.0) | <0.001 |
| Transplant | 3 (0.5) | 3 (0.4) | 6 (2.2) | 0.006 |
| Devices used during admission | ||||
| Central venous catheter | 465 (79.5) | 645 (77.3) | 255 (93.4) | <0.001 |
| Invasive mechanical ventilation | 244 (41.7) | 284 (34.1) | 200 (73.3) | <0.001 |
| Bladder catheter | 520 (88.9) | 804 (96.4) | 266 (97.4) | <0.001 |
| Ventricular shunt | 5 (0.9) | 11 (1.3) | 3 (1.1) | 0.716 |
| Extrarenal filtration | 40 (6.8) | 11 (1.3) | 21 (7.7) | <0.001 |
| Parenteral nutrition | 65 (11.1) | 65 (7.8) | 101 (37.0) | <0.001 |
| ECMO | 0 | 0 | 1 (0.4) | 0.075 |
| Patients with nosocomial infections | ||||
| Ventilator-associated pneumonia | 19 (3.2) | 6 (0.7) | 3 (1.1) | 0.001 |
| Bladder catheter-related urinary infection | 5 (0.9) | 1 (0.1) | 4 (1.5) | 0.025 |
| Bacteremia of unknown origin | 3 (0.5) | 2 (0.2) | 2 (0.7) | 0.490 |
| Catheter-related bacteremia | 3 (0.5) | 2 (0.2) | 3 (1.1) | 0.196 |
| Bacteremia secondary to other locations | 11 (1.9) | 5 (0.6) | 3 (1.1) | 0.079 |
| Urgent surgery during admission | 36 (6.2) | 35 (4.2) | 144 (52.7) | <0.001 |
| Number of antibiotics received in ICU per patient | ||||
| Median (p25–p75) | 2 (1−4) | 1 (0−1) | 2 (1−3) | <0.001 |
| ICU stay | ||||
| Medina (p25–p75) | 5 (3−9) | 3 (2−4) | 5 (3−10) | <0.001 |
| Death | 161 (27.5) | 17 (2.0) | 36 (13.2) | <0.001 |
| Reason for admission in relation to cancer | ||||
| Related to cancer | 362 (62.0) | 786 (94.2) | 209 (76.6) | <0.001 |
| Not related to cancer | 222 (38.0) | 48 (5.8) | 64 (23.4) | |
| Cancer location | ||||
| Head and neck | 37 (6.3) | 80 (9.6) | 13 (4.8) | <0.001 |
| Colon/rectum | 75 (12.8) | 170 (20.4) | 119 (43.6) | |
| Liver/biliary/pancreas | 29 (5.0) | 99 (11.9) | 18 (6.6) | |
| Other digestive | 30 (5.1) | 62 (7.4) | 26 (9.5) | |
| Bronchopulmonary | 88 (15.0) | 74 (8.9) | 6 (2.2) | |
| Renal/urinary | 76 (13.0) | 144 (14.3) | 37 (13.6) | |
| Gynecological | 43 (7.4) | 68 (8.2) | 26 (9.5) | |
| Central nervous system | 17 (2.9) | 105 (12.6) | 10 (3.7) | |
| Leukemia | 52 (8.9) | 0 | 2 (0.7) | |
| Lymphoma | 72 (12.3) | 3 (0.4) | 6 (2.2) | |
| Other hematological | 39 (6.7) | 3 (0.4) | 5 (1.8) | |
| Other types of SO cancer | 27 (4.6) | 26 (3.1) | 5 (1.8) | |
| Year of diagnosis of cancer | ||||
| 2013 | 31 (5.3) | 11 (1.3) | 7 (2.6) | <0.001 |
| 2014 | 42 (7.2) | 32 (3.8) | 8 (2.9) | |
| 2015 | 43 (7.4) | 36 (4.3) | 15 (5.5) | |
| 2016 | 45 (7.7) | 43 (5.2) | 22 (8.1) | |
| 2017 | 142 (24.3) | 158 (18.9) | 43 (15.8) | |
| 2018 | 282 (48.2) | 554 (66.4) | 178 (65.2) | |
| Oncological treatment of the patient on admission to the ICU | ||||
| Pending start of treatment | 136 (23.3) | 390 (46.8) | 119 (43.6) | <0.001 |
| Neoadjuvant therapy | 33 (5.7) | 167 (20.0) | 29 (10.6) | |
| Adjuvant therapy | 56 (9.6) | 50 (6.0) | 17 (6.2) | |
| CT-RT and radical intent | 73 (12.5) | 29 (3.5) | 15 (5.5) | |
| Treatment of metastatic disease | 45 (7.7) | 20 (2.4) | 9 (3.3) | |
| Symptomatic treatment | 57 (9.8) | 45 (5.4) | 25 (9.2) | |
| Allogenic bone marrow transplant | 9 (1.5) | 0 | 0 | |
| Autologous bone marrow transplant | 9 (1.5) | 0 | 0 | |
| Chemotherapy for acute leukemia | 41 (7.0) | 12 (1.4) | 2 (0.7) | |
| Others | 125 (21.4) | 121 (14.5) | 57 (20.9) | |
| Development of neutropenia in ICU | 69 (11.8) | 2 (0.2) | 7 (2.6) | <0.001 |
| Chemotherapy in ICU | 27 (4.6) | 1 (0.1) | 1 (0.4) | <0.001 |
| Tumor lysis syndrome | 13 (2.2) | 0 | 0 | <0.001 |
| Limitation of life support treatments | 82 (14.0) | 6 (0.7) | 10 (3.7) | <0.001 |
| Probable aspergillosis | 13 (2.2) | 1 (0.1) | 1 (0.4) | <0.001 |
APACHE: Acute Physiology, Age and Chronic Health Evaluation; SD: standard deviation; ECMO: extracorporeal membrane oxygenation; COPD: chronic obstructive pulmonary disease; SO: solid organ; p25–p75: percentiles 25 and 75; CT-RT: chemotherapy-radiotherapy; SAPS: Simplified Acute Physiology Score.
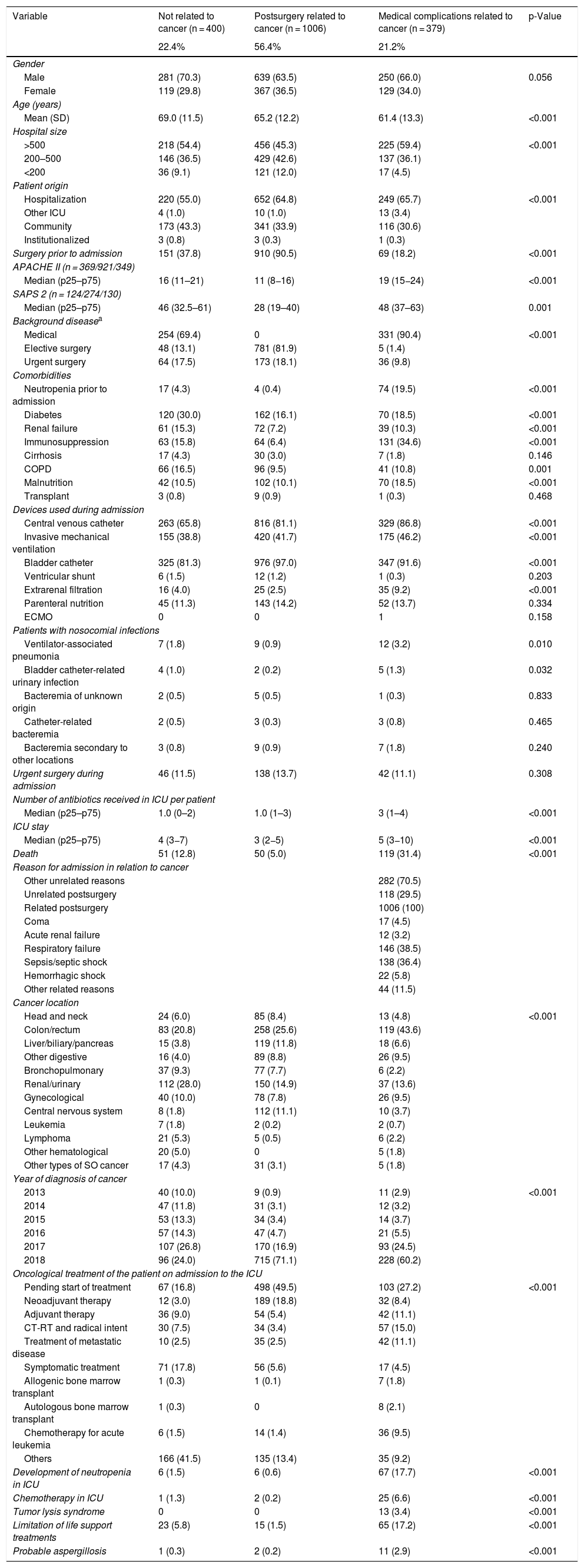
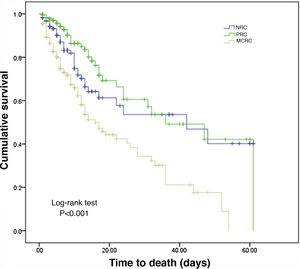
Another epidemiological approach involves taking into account the reason for admission in relation to the neoplastic disease itself. Three groups were established: no relation to cancer (NRC) (n = 400); postsurgery related to cancer (PRC) (n = 1006); and medical complications related to cancer (MCRC) (n = 379) — with the inclusion of patients as described in the methodology. This latter group consisted of younger individuals, in more serious condition, with predominantly background medical disease, and with a greater use of devices such as mechanical ventilation (46.2%) or extrarenal filtration (9.2%). Table 3 describes the characteristics of these patients, including the location of the tumor, the year of cancer diagnosis, and the antineoplastic treatment received. Of note is the absence of treatment up until the time of admission in most of the PRC patients (49.5%). In contrast, having received treatments different from those of the above groups was more frequent among the NRC patients (41.5%). Neutropenia during admission, chemotherapy in the ICU or tumor lysis syndrome proved more frequent in MCRC patients. A relevant finding in this latter group is the proportion of patients with some form of limitation of therapeutic effort (17.2%) versus the other groups (NRC: 5.8% and PRC: 1.5%; p < 0.001). Probable aspergillosis was diagnosed in 2.9% of the MCRC patients.
Comparative analysis of patients with cancer according to reason for admission to the ICU.
| Variable | Not related to cancer (n = 400) | Postsurgery related to cancer (n = 1006) | Medical complications related to cancer (n = 379) | p-Value |
|---|---|---|---|---|
| 22.4% | 56.4% | 21.2% | ||
| Gender | ||||
| Male | 281 (70.3) | 639 (63.5) | 250 (66.0) | 0.056 |
| Female | 119 (29.8) | 367 (36.5) | 129 (34.0) | |
| Age (years) | ||||
| Mean (SD) | 69.0 (11.5) | 65.2 (12.2) | 61.4 (13.3) | <0.001 |
| Hospital size | ||||
| >500 | 218 (54.4) | 456 (45.3) | 225 (59.4) | <0.001 |
| 200–500 | 146 (36.5) | 429 (42.6) | 137 (36.1) | |
| <200 | 36 (9.1) | 121 (12.0) | 17 (4.5) | |
| Patient origin | ||||
| Hospitalization | 220 (55.0) | 652 (64.8) | 249 (65.7) | <0.001 |
| Other ICU | 4 (1.0) | 10 (1.0) | 13 (3.4) | |
| Community | 173 (43.3) | 341 (33.9) | 116 (30.6) | |
| Institutionalized | 3 (0.8) | 3 (0.3) | 1 (0.3) | |
| Surgery prior to admission | 151 (37.8) | 910 (90.5) | 69 (18.2) | <0.001 |
| APACHE II (n = 369/921/349) | ||||
| Median (p25–p75) | 16 (11–21) | 11 (8−16) | 19 (15−24) | <0.001 |
| SAPS 2 (n = 124/274/130) | ||||
| Median (p25–p75) | 46 (32.5–61) | 28 (19–40) | 48 (37–63) | 0.001 |
| Background diseasea | ||||
| Medical | 254 (69.4) | 0 | 331 (90.4) | <0.001 |
| Elective surgery | 48 (13.1) | 781 (81.9) | 5 (1.4) | |
| Urgent surgery | 64 (17.5) | 173 (18.1) | 36 (9.8) | |
| Comorbidities | ||||
| Neutropenia prior to admission | 17 (4.3) | 4 (0.4) | 74 (19.5) | <0.001 |
| Diabetes | 120 (30.0) | 162 (16.1) | 70 (18.5) | <0.001 |
| Renal failure | 61 (15.3) | 72 (7.2) | 39 (10.3) | <0.001 |
| Immunosuppression | 63 (15.8) | 64 (6.4) | 131 (34.6) | <0.001 |
| Cirrhosis | 17 (4.3) | 30 (3.0) | 7 (1.8) | 0.146 |
| COPD | 66 (16.5) | 96 (9.5) | 41 (10.8) | 0.001 |
| Malnutrition | 42 (10.5) | 102 (10.1) | 70 (18.5) | <0.001 |
| Transplant | 3 (0.8) | 9 (0.9) | 1 (0.3) | 0.468 |
| Devices used during admission | ||||
| Central venous catheter | 263 (65.8) | 816 (81.1) | 329 (86.8) | <0.001 |
| Invasive mechanical ventilation | 155 (38.8) | 420 (41.7) | 175 (46.2) | <0.001 |
| Bladder catheter | 325 (81.3) | 976 (97.0) | 347 (91.6) | <0.001 |
| Ventricular shunt | 6 (1.5) | 12 (1.2) | 1 (0.3) | 0.203 |
| Extrarenal filtration | 16 (4.0) | 25 (2.5) | 35 (9.2) | <0.001 |
| Parenteral nutrition | 45 (11.3) | 143 (14.2) | 52 (13.7) | 0.334 |
| ECMO | 0 | 0 | 1 | 0.158 |
| Patients with nosocomial infections | ||||
| Ventilator-associated pneumonia | 7 (1.8) | 9 (0.9) | 12 (3.2) | 0.010 |
| Bladder catheter-related urinary infection | 4 (1.0) | 2 (0.2) | 5 (1.3) | 0.032 |
| Bacteremia of unknown origin | 2 (0.5) | 5 (0.5) | 1 (0.3) | 0.833 |
| Catheter-related bacteremia | 2 (0.5) | 3 (0.3) | 3 (0.8) | 0.465 |
| Bacteremia secondary to other locations | 3 (0.8) | 9 (0.9) | 7 (1.8) | 0.240 |
| Urgent surgery during admission | 46 (11.5) | 138 (13.7) | 42 (11.1) | 0.308 |
| Number of antibiotics received in ICU per patient | ||||
| Median (p25–p75) | 1.0 (0–2) | 1.0 (1–3) | 3 (1–4) | <0.001 |
| ICU stay | ||||
| Median (p25–p75) | 4 (3−7) | 3 (2−5) | 5 (3−10) | <0.001 |
| Death | 51 (12.8) | 50 (5.0) | 119 (31.4) | <0.001 |
| Reason for admission in relation to cancer | ||||
| Other unrelated reasons | 282 (70.5) | |||
| Unrelated postsurgery | 118 (29.5) | |||
| Related postsurgery | 1006 (100) | |||
| Coma | 17 (4.5) | |||
| Acute renal failure | 12 (3.2) | |||
| Respiratory failure | 146 (38.5) | |||
| Sepsis/septic shock | 138 (36.4) | |||
| Hemorrhagic shock | 22 (5.8) | |||
| Other related reasons | 44 (11.5) | |||
| Cancer location | ||||
| Head and neck | 24 (6.0) | 85 (8.4) | 13 (4.8) | <0.001 |
| Colon/rectum | 83 (20.8) | 258 (25.6) | 119 (43.6) | |
| Liver/biliary/pancreas | 15 (3.8) | 119 (11.8) | 18 (6.6) | |
| Other digestive | 16 (4.0) | 89 (8.8) | 26 (9.5) | |
| Bronchopulmonary | 37 (9.3) | 77 (7.7) | 6 (2.2) | |
| Renal/urinary | 112 (28.0) | 150 (14.9) | 37 (13.6) | |
| Gynecological | 40 (10.0) | 78 (7.8) | 26 (9.5) | |
| Central nervous system | 8 (1.8) | 112 (11.1) | 10 (3.7) | |
| Leukemia | 7 (1.8) | 2 (0.2) | 2 (0.7) | |
| Lymphoma | 21 (5.3) | 5 (0.5) | 6 (2.2) | |
| Other hematological | 20 (5.0) | 0 | 5 (1.8) | |
| Other types of SO cancer | 17 (4.3) | 31 (3.1) | 5 (1.8) | |
| Year of diagnosis of cancer | ||||
| 2013 | 40 (10.0) | 9 (0.9) | 11 (2.9) | <0.001 |
| 2014 | 47 (11.8) | 31 (3.1) | 12 (3.2) | |
| 2015 | 53 (13.3) | 34 (3.4) | 14 (3.7) | |
| 2016 | 57 (14.3) | 47 (4.7) | 21 (5.5) | |
| 2017 | 107 (26.8) | 170 (16.9) | 93 (24.5) | |
| 2018 | 96 (24.0) | 715 (71.1) | 228 (60.2) | |
| Oncological treatment of the patient on admission to the ICU | ||||
| Pending start of treatment | 67 (16.8) | 498 (49.5) | 103 (27.2) | <0.001 |
| Neoadjuvant therapy | 12 (3.0) | 189 (18.8) | 32 (8.4) | |
| Adjuvant therapy | 36 (9.0) | 54 (5.4) | 42 (11.1) | |
| CT-RT and radical intent | 30 (7.5) | 34 (3.4) | 57 (15.0) | |
| Treatment of metastatic disease | 10 (2.5) | 35 (2.5) | 42 (11.1) | |
| Symptomatic treatment | 71 (17.8) | 56 (5.6) | 17 (4.5) | |
| Allogenic bone marrow transplant | 1 (0.3) | 1 (0.1) | 7 (1.8) | |
| Autologous bone marrow transplant | 1 (0.3) | 0 | 8 (2.1) | |
| Chemotherapy for acute leukemia | 6 (1.5) | 14 (1.4) | 36 (9.5) | |
| Others | 166 (41.5) | 135 (13.4) | 35 (9.2) | |
| Development of neutropenia in ICU | 6 (1.5) | 6 (0.6) | 67 (17.7) | <0.001 |
| Chemotherapy in ICU | 1 (1.3) | 2 (0.2) | 25 (6.6) | <0.001 |
| Tumor lysis syndrome | 0 | 0 | 13 (3.4) | <0.001 |
| Limitation of life support treatments | 23 (5.8) | 15 (1.5) | 65 (17.2) | <0.001 |
| Probable aspergillosis | 1 (0.3) | 2 (0.2) | 11 (2.9) | <0.001 |
APACHE: Acute Physiology, Age and Chronic Health Evaluation; SD: standard deviation; ECMO: extracorporeal membrane oxygenation; COPD: chronic obstructive pulmonary disease; SO: solid organ; p25–p75: percentiles 25 and 75; CT-RT: chemotherapy-radiotherapy; SAPS: Simplified Acute Physiology Score.
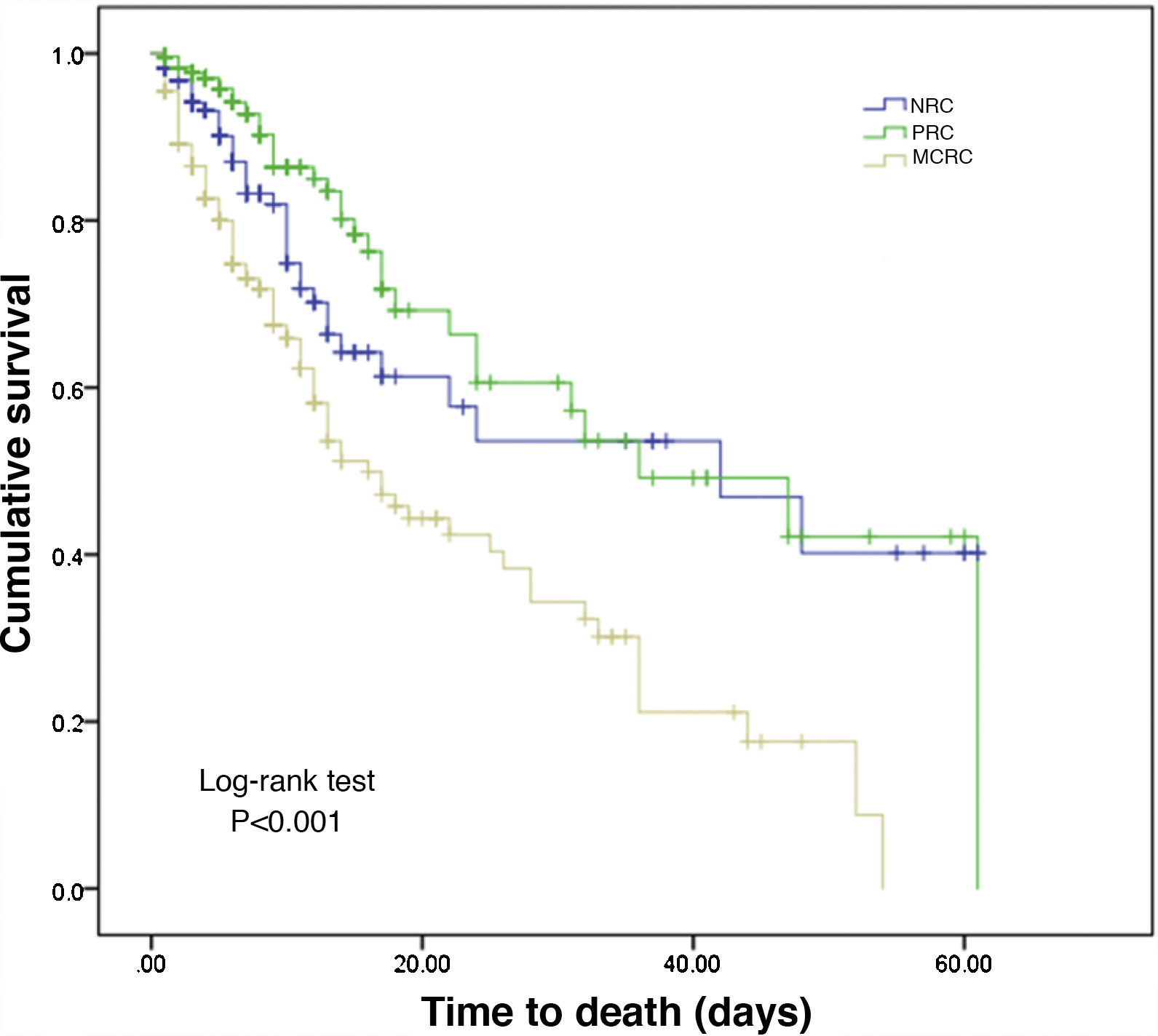
The duration of ICU stay was longer in the MCRC patients than in those belonging to the other groups, and in-ICU mortality was also significantly greater (31.4%) than in the NRC (12.8%) or PRC patients (5%) (p < 0.001). In terms of overall survival, the MCRC patients were clearly differentiated from the other two groups (log-rank test, p < 0.001), as can be seen from the Kaplan–Meier curves shown in Fig. 3.
DiscussionThe present study provides the first description in Spain of the epidemiology of patients with solid organ tumors or hematological malignancies requiring admission to the ICU. Fifteen percent of all patients admitted to the ICU have cancer, though there is marked variability in this percentage depending on the type of Unit involved — a circumstance that reflects the heterogeneity of the Spanish ICUs. The conduction of this multicenter study, based on the Units participating in the ENVIN registry, has afforded a very precise image of the case-mix of the cancer patients admitted to the ICU, due both to the large number of participating Units and to their widespread geographical distribution. None of the participating Units were monographic cancer patient ICUs. The multicenter and voluntary nature of the registry implied that not all the Units participating in the ENVIN registry completed the data on cancer patients, and thus that not all the possible patients have been recorded. The utilization of the arbitrary criterion of securing the complete registry of at least 80% of the patients with cancer reduced the number of participating ICUs, but in our opinion it affords a quite accurate idea of the epidemiological reality of cancer patients requiring admission to intensive care in our country.
Although it was not the objective of our study to analyze factors related to mortality (an aspect that will be examined by another article), the mortality rate in the ICU among cancer patients was significantly higher than in patients without cancer (12.3% versus 8.5%; p < 0.001). However, due to the heterogeneity of the cancer patients, this observation says little of the factors influencing such mortality. The Kaplan–Meier curves offer a good idea of mortality differences, but do not represent more than a point in time (ICU stay) in the course of the neoplastic disease. They consequently must be correlated to their true value in relation to the number of live patients discharged from the ICU. No in-hospital mortality data were available, as the reporting of such information is not mandatory in the ENVIN registry.
Among the cancer patients, those admitted to the ICU for postsurgery care represented 62% of the total. This figure is the same as that recorded in the European series derived from the SOAP trial,16 and similar to the percentages obtained in the multicenter Dutch18 and Brazilian studies19 (56% and 64%, respectively). In this group of patients, and in the same way as in the global population of critical patients,29 a distinction is made between those who are admitted to the ICU in the postoperative period of elective surgery (less severe cases, with shorter stays and lesser mortality) and those who require admission following urgent surgery (more severe cases, with stays and mortality rates intermediate between those of the elective surgery patients and patients with background medical disease). Some authors have only studied the admission of non-elective cases, which allows for a certain unification of the case-mix,17,20 though the distinction between medical cases (40.7%) and urgent surgical cases (59.3%) remains considerable — with a poorer prognosis among the former.
The patients admitted due to medical causes (representing 34.6% of all the patients with cancer) constituted a very heterogeneous group, with differences between those presenting solid organ tumors (72.1%) and those with hematological neoplasms (27.9%). The proportion of hematological patients admitted to the ICU in Spain is greater than that recorded in other series, where the figures range between 14.6%–17%.16,19
The mortality rate among the medical cases with cancer (27.5%) was clearly higher than in the other two groups (2% and 13.2% for elective and urgent surgery, respectively), as has also been reported in other epidemiological series.19 The in-ICU mortality rate in the multicenter Dutch study17 was 30.4% for medical cases and 16.2% for surgical cases. On the other hand, it is notorious that the proportion of patients in which the limitation of therapeutic effort was decided proved clearly higher (14%) in the group of medical patients versus the other patient groups — thus identifying a population of more complex patients with more serious acute disease. It would be interesting to examine the influence of the staging of cancer disease in this decision.
It is of interest to describe the reasons for admission to the ICU and their relation to cancer. This relationship was strong among the surgical patients (94.2% of the elective surgery patients and 76.6% of the urgent surgery cases), and less manifest among the medical cases — with a high percentage of patients being admitted to the ICU due to reasons not directly related to cancer (38%), and which represent intercurrent processes that are seen in both oncological patients and in the non-oncological population.
Respiratory failure (38.5%) and sepsis/septic shock (36.4%) were the most frequent causes of admission to the ICU in the medical complications related to cancer (MCRC) group. This observation is not surprising, since the use of invasive mechanical ventilation is more frequent in these patients, and vasopressor drug administration (not recorded in this study) is probably also more common, as suggested by the literature.16,19,21 The global patients admitted due to reasons related to cancer presented a mortality rate of 31.4%, which demonstrates the importance of organ failure in the clinical course of these patients, which constitute a subgroup of special individuals due to their high mortality and use of resources — generating debate regarding the possible futility of their admission to the ICU.
With regard to the cancer treatment received before admission to the ICU, we recorded a notoriously high percentage of symptomatic treatments (17.8%) or other treatments (41.5%) among those patients admitted to the ICU due to reasons unrelated to cancer. These patients possibly correspond to two extremes: those who receive palliative treatment and those free of active disease who are not receiving specific treatments, and who are admitted to the ICU for other quite different reasons.
The present study has a number of limitations. Assessing cancer stage and evaluating its influence in deciding or not deciding treatments is a complex matter. Although an attempt has been made to encompass all the therapeutic possibilities as a continuity from the patient pending the start of treatment to the treatment of metastatic disease, this aspect is not easy to define at the point in time represented by admission to the ICU. Perhaps more simple definitions for the type of cancer (local or metastatic for solid organ tumors; high or low malignancy for hematological neoplasms) would have allowed for better grouping of the patients and easier analysis,19 but there would always remain a percentage of patients in which the evolutive stage of the disease cannot be determined. It also must be taken into account that what we documented was in-ICU mortality, not in-hospital mortality or mortality at 90 days, which would have been more correct in relation to the course of the neoplastic disease process.
The percentage of patients with cancer and nosocomial infections, including aspergillosis, was low in all the analyzed groups, though we did not calculate the rates related to the use of devices that favor infection (as registered in the ENVIN); no conclusions therefore can be drawn in this regard. In any case, nosocomial infections in these patients do not appear to constitute a problem much different from that seen in the general population without malignant disease.
The choice of 5 years as the limit in defining a personal history of cancer is arbitrary. It is possible that some of these patients were “healed” of their cancer at the time of admission to the ICU. However, we adopted this criterion because it is the definition used in the ENVIN, and due to the difficulties that may be encountered in deciding whether a cancer has healed or not in patients admitted to the ICU for other reasons. It is also complicated to determine whether the reasons for admission to the ICU are related to the neoplasm or not. This seems clear in the extreme scenarios (postsurgery or septic shock in a patient with profound post-chemotherapy neutropenia), but it might not be so clear in intermediate patients. On the other hand, the multiple comparisons made identified statistically significant differences as a consequence of the sample size. Some of these differences are of scant clinical significance, however, and would require more detailed post hoc analysis.
In conclusion, the present multicenter study describes the epidemiology of patients with a recent history of cancer or who are admitted for the treatment of cancer or its complications. There is great variability in the percentage of cancer patients in the different ICUs in Spain, with a predominance of patients in the postoperative period of elective surgery (46.7%). The global mortality rate among the cancer patients was 12.3%, though a more seriously ill patient population with greater mortality is identified. Specifically, the mortality rate among the patients with background medical disease was 27.5%, while in those subjects admitted due to medical complications related to cancer, the mortality rate was even higher (31.4%). More specific studies are needed on the mortality-related factors in these populations.
AuthorshipPMOA, FAL and RGC are coordinators of the ENVIN registry. JGM, FGV and CBZ form part of the SEMICYUC-SEOM collaborative working group. RDN, CRS and IAA are principal investigators at their hospitals and contributed a larger number of cases. The study was designed by the SEMICYUC-SEOM working group. The manuscript was written by PMOA and corrected by the rest of the authors, who agree to its contents.
Financial supportThe ENVIN-HELICS registry was partially funded during the year 2018 by the Spanish Medicines Agency (Agencia Española del Medicamento y Productos Sanitarios [AEMPS]).
Conflicts of interestThe authors declare that they have no conflicts of interest in relation to the present manuscript.
This work would not have been possible without the participation of all the collaborators (physicians and nurses) of the ENVIN-HELICS registry in the different participating ICUs. Thanks are also due to Drs. Sonia Uriona and Yolima Cosio for administrative and technical secretarial support of the ENVIN-HELICS registry. On the other hand, we thank the secretarial staff of the SEMICYUC, particularly Miguel Angel Ferrero, who is in charge of the ONCOENVIN database. Likewise, thanks are due to Sergi Mojal for his collaboration in the methodology and statistical analysis.
Raquel Durá Navarro, Hospital General Universitario de Valencia (U. Polivalente). María Carmen Ruano Suarez, Hospital de Cruces (U. Reanimación), Baracaldo, Vizcaya. Inmaculada Alonso Araujo, Hospital General Virgen del Rocío, Seville. Ángel Arenzana Seisdedos, Hospital Universitario Virgen Macarena, Seville. Alberto Córdoba López, Complejo Hospitalario Universitario de Badajoz (UCI 1). Nuria Camino Redondo, Hospital de Torrejón, Torrejon de Ardoz, Madrid. Sandra Barbadillo Ansorregui, Hospital General de Catalunya, Sant Cugat del Vallés, Barcelona. Lorena Mouriz Fernández, Hospital Universitario Lucus Augusti (U. Reanimación), Lugo. Maria Elena Vilas Otero, Complexo Hospitalario Universitario de Vigo H. Álvaro Cunqueiro (REA 2), Vigo. José Antonio Márquez Alonso, Hospital Rey Juan Carlos, Móstoles, Madrid. Adoración Gema Bueno Blázquez, Clínica Moncloa, Madrid. Ana Abella Alvarez, Hospital del Henares, Coslada, Madrid. Joaquín Lobo Palanco, Complejo Hospitalario de Navarra (UCI-A), Pamplona. Luis Cofiño Castañeda, Hospital Universitario Central de Asturias (U. Polivalente), Oviedo, Asturias. J.C. Montejo González, Hospital Universitario 12 de Octubre, U. Polivalente, Madrid. Miguel Ángel García García, Hospital de Sagunto, Valencia. María Dolores Sandar Núñez, Hospital Jerez de la Frontera, Cádiz. María Teresa Tebar Soto, Hospital de Basurto, U. Polivalente, Bilbao, Vizcaya. Rafael Cabadas Avión, Hospital Povisa, Vigo, Pontevedra. Ricardo Gimeno Costa, Hospital Universitario y Politécnico La Fe (U. Médica), Valencia. José Ángel Berezo García, Hospital Universitario Río Hortega, Valladolid. Fernando García López, Hospital General Universitario de Albacete. Blanca López Matamala, Hospital Universitario del Tajo, Aranjuez, Madrid. Asunción Colomar Ferrá, Hospital Universitario Son Espases, Palma de Mallorca. María Sopetrán Rey García, Complejo Asistencial de Segovia. Belén Cidoncha Calderón, Hospital Don Benito-Villanueva, Badajoz. Sara Alcántara Carmona, Hospital Universitario Puerta de Hierro Majadahonda, Madrid. Eva Manteiga Riestra, Hospital Infanta Cristina, Parla, Madrid. Bernardo Gil Rueda, Hospital General Universitario Morales Meseguer, Murcia. Carlos Gallego González, Hospital Militar Gómez Ulla, Madrid. Roberto Jiménez Sánchez, Hospital General Universitario Santa Lucía, Cartagena, Murcia. Ismael López de Toro Martín-Consuegra, Hospital Virgen de la Salud (U. Polivalente), Toledo. Jessica Souto Higueras, Hospital Sanitas CIMA de Barcelona. Arantxa Lander Azcona, Hospital General San Jorge, Huesca. José María Fuster Lozano, Clínica Vistahermosa, HLA Grupo Hospitalario, Alicante. Paula Vera Artázcoz, Hospital de Sant Pau (U. Polivalente), Barcelona. María José Castro Orjales, Complejo Hospitalario Universitario de Ferrol. H. Arquitecto Marcide, La Coruña. María José Asensio Martín, Hospital Universitario La Paz (U. Polivalente), Madrid. María Antonia Estecha Foncea, Hospital Universitario Virgen de la Victoria, Málaga. Roberto Reig Valero, Hospital General de Castellón. Jesús Priego Sanz, Complexo Hospitalario Universitario de Ourense (U. Polivalente), Susana Sancho Chinesta, Hospital Doctor Peset, Valencia. Jordi Vallés Daunis, Hospital Parc Tauli, Sabadell, Barcelona. Ana Isabel Ezpeleta Galindo, Hospital Royo Villanova, Zaragoza. Braulio Álvarez Martínez, Hospital El Bierzo, Ponferrada, León. Felipe Bobillo de Lamo, Hospital Clínico Universitario de Valladolid, U. Polivalente. Antoni Margarit Ribas, Hospital Nuestra Señora de Meritxell, Escaldes-Engordany, Andorra. Pedro M. Olaechea Astigarraga, Hospital de Galdakao (U. Polivalente), Vizcaya. Juan Carlos Ballesteros Herráez, Hospital Clínico de Salamanca. María Teresa Saldaña Fernández, Hospital Universitario de Fuenlabrada, Madrid. Ángel Sánchez Miralles, Hospital de Sant Joan, Alicante. Rosario Amaya Villar, Hospital de Rehabilitación y Traumatología Virgen del Rocío, Seville. Juan Fajardo López-Cuervo, Clínica Santa Isabel, Seville. Antonia Socias, Hospital Son Llàtzer, Palma de Mallorca. Alfons Bonet Saris, Clínica Girona. Ana María Díaz Lamas, Complexo Hospitalario Universitario de A Coruña (UCI 5). José Ramón Iruretagoyena Amiano, Hospital de Cruces (U. Polivalente), Baracaldo, Vizcaya. Ingrid Acosta Rivera, Clínica Ruber de Madrid. María Cerón García, Hospital Vega Baja de Orihuela, Alicante. Susana Moradillo González, Hospital Río Carrión, Complejo Hospitalario de Palencia. Paula Rodríguez Pedreira, Hospital Quirónsalud, Barcelona. Eduardo Palencia Herrejón, Hospital Infanta Leonor, Madrid. Carlos López Núñez, Hospital Clínico Universitario Lozano Blesa (U. Médica), Zaragoza. Margarita Mas Lodo, Hospital General, Móstoles, Madrid. Juan Carlos Pardo Talavera, Hospital Quirón Murcia. María Luisa Mora, Hospital Universitario de Canarias (UPCC), Santa Cruz de Tenerife. Ricard Ferrer Roca, Centro Médico Delfos, Barcelona. Eugenia de La Fuente Óconnor, Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Madrid. Miguel Sánchez García, Hospital Clínico Universitario San Carlos (U. Traumatología), Madrid. Carmen Blanco Huelga, Hospital Marqués de Valdecilla (UCI 1), Santander. María Ángeles Garijo Catalina, Hospital Virgen de la Luz, Cuenca. Adoración Alcalá López, Hospital General Universitario de Elche, Alicante. Marta Ugalde Gutierrez, Hospital de Cruces (U. Quemados), Baracaldo, Vizcaya. María Rosa Navarro Ruiz, Hospital Universitario los Arcos del Mar Menor, San Javier, Murcia. María José Román Millan, Hospital de la Merced, Osuna, Seville. Pedro Lara Aguayo, Hospital Infanta Margarita, Cabra, Córdoba. María Herreros Gonzalo, Hospital La Mancha Centro, Alcázar de San Juan, Ciudad Real. Laura Claverias Cabrera, Hospital de Tortosa Verge de la Cinta, Tarragona. José Martos López, Hospital Vithas La Salud de Granada. María Concepción Valdovinos Mahave, Hospital Obispo Polanco, Teruel. Daniel Fontaneda López, Hospital de León (U. Polivalente). María Matachana Martínez, Hospital Juan Cardona, Ferrol, La Coruña. Esther García Sánchez, Hospital Universitario del Sureste, Arganda del Rey, Madrid. Carmen Santarrufina Lluch, Hospital Comarcal de Vinaròs, Castellón. Rafael Garcés González, Hospital de la Ribera, Alzira, Valencia. Sonia Gallego Lara, Hospital San Juan de Dios del Aljarafe, Seville. Pilar Martinez Trivez, Hospital de Barbastro, Huesca. Cecília Vilanova Pàmies, Hospital Mateu Orfila, Menorca. Celina Llanos Jorge, Hospital Quirónsalud, Santa Cruz de Tenerife. María José Asensio Martín, Hospital Universitario La Paz (U. Quemados), Madrid. Juan Carlos Montejo Gonzalez, Hospital Universitario 12 de Octubre (U. Trauma y Emergencia), Madrid. Enrique Alemparte Pardavila, Complexo Hospitalario Universitario A Coruña (UCI 6).
Please cite this article as: Olaechea Astigarraga PM, Álvarez Lerma F, Beato Zambrano C, Gimeno Costa R, Gordo Vidal F, Durá Navarro R, et al. Epidemiología y pronóstico de los pacientes con antecedentes de neoplasia ingresados en las Unidades de Cuidados Intensivos. Estudio multicéntrico observacional. Med Intensiva. 2021;45:332–346.
The list of supervisors and Units participating in the ONCOENVIN trial, ordered by the number of patients contributed to the epidemiological study, is found in the Appendix A.