To describe the characteristics and evolution of patients with bronchiolitis admitted to a pediatric intensive care unit, and compare treatment pre- and post-publication of the American Academy of Pediatrics clinical practice guide.
DesignA descriptive and observational study was carried out between September 2010 and September 2017.
SettingPediatric intensive care unit.
PatientsInfants under one year of age with severe bronchiolitis.
InterventionsTwo periods were compared (2010–14 and 2015–17), corresponding to before and after modification of the American Academy of Pediatrics guidelines for the management of bronchiolitis in hospital.
Main variablesPatient sex, age, comorbidities, severity, etiology, administered treatment, bacterial infections, respiratory and inotropic support, length of stay and mortality.
ResultsA total of 706 patients were enrolled, of which 414 (58.6%) males, with a median age of 47 days (IQR 25–100.25). Median bronchiolitis severity score (BROSJOD) upon admission: 9 points (IQR 7–11). Respiratory syncytial virus appeared in 460 (65.16%) patients. The first period (2010–14) included 340 patients and the second period (2015–17) 366 patients. More adrenalin and hypertonic saline nebulizations and more corticosteroid treatment were administered in the second period. More noninvasive ventilation and less conventional mechanical ventilation were used, and less inotropic support was needed, with no significant differences. The antibiotherapy rate decreased significantly (p=0.003).
ConclusionsDespite the decrease in antibiotherapy, the use of nebulizations and glucocorticoids in these patients should be limited, as recommended by the guide.
Describir las características y la evolución de los pacientes con bronquiolitis ingresados en una unidad de cuidados intensivos pediátricos. Comparar el tratamiento administrado pre y pospublicación de la guía de práctica clínica de la Academia Americana de Pediatría.
DiseñoEstudio descriptivo y observacional realizado entre septiembre de 2010 y septiembre de 2017.
ConfiguraciónUnidad de cuidados intensivos pediátricos.
PacientesMenores de un año con bronquiolitis grave.
IntervencionesSe compararon 2 períodos (2010-14 y 2015-17), antes y después de la modificación del protocolo de manejo de la bronquiolitis en el hospital, según las guías de la Academia Americana de Pediatría.
Principales variablesSexo, edad, comorbilidades, gravedad, etiología, tratamiento administrado, infecciones bacterianas, soporte respiratorio e inotrópico, estancia y mortalidad.
ResultadosSe recogieron 706 pacientes, 414 (58,6%) varones, con una mediana de edad de 47 días (RIC 25-100,25). Mediana de escala de gravedad de bronquiolitis (BROSJOD) al ingreso: 9 puntos (RIC 7-11). La etiología por virus respiratorio sincitial se dio en 460 (65,16%) pacientes. El primer período (2010-14) incluyó 340 pacientes y el segundo (2015-17), 366 pacientes. En el segundo período se administraron más nebulizaciones de adrenalina y suero salino hipertónico, y más tratamiento con corticoides. Se usó más ventilación no invasiva y menos ventilación mecánica convencional y precisaron menos soporte inotrópico, sin diferencias significativas. La tasa de antibioterapia disminuyó de forma estadísticamente significativa (p=0,003).
ConclusionesPese a la disminución en la antibioterapia, se debería limitar la utilización de nebulizaciones y corticoides en estos pacientes, como recomienda la guía.
Acute bronchiolitis (AB) is the most common cause of lower respiratory tract infection in nursing infants, and of hospital admission in infants under one year of age.1,2 The respiratory syncytial virus (RSV) is the most frequent causal microorganism, though many others can also cause the disorder, with different degrees of severity.3–5
Acute bronchiolitis is characterized as the first episode of breathing difficulty in nursing infants under 24 months of age, preceded by an upper airway infection, and commonly occurring in an epidemic period.6 Consensus is lacking regarding the clinical definition of acute bronchiolitis in children.7,8 Studies on the admission rates attributable to the disorder are scarce, though it has been reported that 2–3% of all patients with acute bronchiolitis require hospital admission, and of these, 3–11% require admission to the Pediatric Intensive Care Unit (PICU) – though this figure reaches 50% in populations with associated risk factors.9,10
Few therapeutic measures of established efficacy in application to acute bronchiolitis are available. Many studies have described great variability in patient management.11,12 Treatment is merely supportive, and the adoption of a conservative management approach seems adequate in most children, particularly in the youngest patients. The fact that most cases evolve favorably over time, independently of the chosen treatment option, probably explains why we still use certain drugs with no demonstrated clinical benefits, based on professional or institutional preferences.13–17 Bronchiolitis generates an important problem, since most drug treatments have been shown to be of little help, and the current guides recommend that drug treatment be restricted in most cases. As a result, there is widespread variability in reference to both over- and under-treatment. Evaluation of adherence to the international recommendations is very important. In this respect, bronchiolitis constitutes a challenge for the general management of each patient, specific treatment, and ventilatory support.
The present study comprises an epidemiological analysis and study of the clinical characteristics, treatment and outcome of patients with severe acute respiratory failure secondary to bronchiolitis requiring admission to a PICU. On the other hand, comparisons were made between two time periods – before and after publication of the clinical practice guide (CPG) of the American Academy of Pediatrics (AAP)18 — to determine whether the new recommendations have been followed in our Unit.
Patients and methodsA post hoc, descriptive observational study was carried out, involving the retrospective analysis of a prospective database of all patients under one year of age with severe acute bronchiolitis admitted to the PICU of a tertiary hospital during the period between September 2010 and September 2017. The hospital is a reference center with 320 beds that covers 30% of all hospitalized patients in the region (Spanish Autonomous Community). Bronchiolitis was diagnosed based on the classical criteria.6 As sole exclusion criterion, we excluded those infants in which parent informed consent to inclusion in the database could not be obtained.
In the year 2010 our Unit created a prospective registry of patients diagnosed with bronchiolitis. The entered data are dissociated in order to ensure patient anonymity, and access to the database is controlled by means of a password.
Epidemiological, clinical and microbiological information was collected: patient gender, age upon admission, origin, comorbidities (premature or ex-premature infants <37 gestational weeks, bronchopulmonary dysplasia as based on the need for oxygen therapy >28 days, congenital heart disease or neurological disorders), Pediatric Risk Score of Mortality III (PRISM-III),19 Bronchiolitis Score of Sant Joan de Deu (BROSJOD),20 cause of acute respiratory failure, treatment received, respiratory and inotropic support, need for invasive devices, duration of admission to the PICU, global hospital stay and mortality.
A nasopharyngeal aspirate was obtained from all patients for polymerase chain reaction (PCR) testing for respiratory viruses (the routine procedure in our Unit). Our microbiology laboratory determines the presence of RSV rhinovirus, metapneumovirus, virus influenza and parainfluenza, adenovirus, coronavirus, enterovirus, as well as Bordetella pertussis and parapertussis. In the context of invasive bacterial infection, we considered the presence of pneumonia, urinary tract infection and sepsis, which were defined according to the international guides.21 Only confirmed cases were included. Nosocomial bronchiolitis was regarded as bronchiolitis manifesting in patients admitted to the hospital for over 72h at the time of the diagnosis, while the rest of cases were considered to correspond to community-acquired bronchiolitis. We included blood, urine and cerebrospinal fluid (CSF) culture samples in the case of patients with suspected bacterial infection, and nasopharyngeal or tracheal aspirates in those patients with suspected bacterial pneumonia (consistent chest X-ray findings, fever ≥38°C, C-reactive protein ≥70mg/dl and/or procalcitonin ≥1ng/ml).
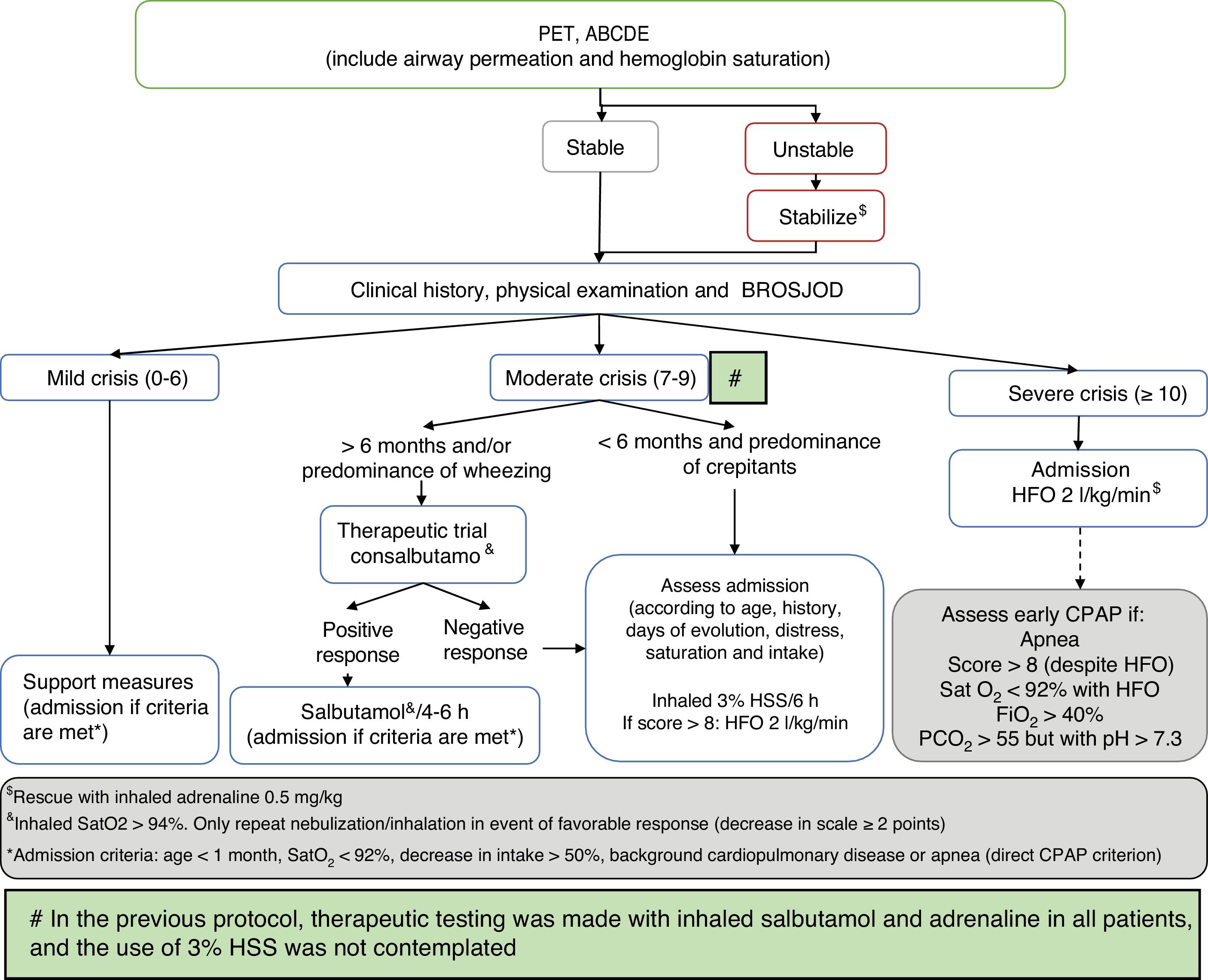
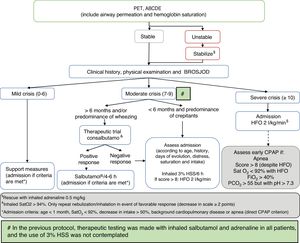
Comparison was made of the described data corresponding to two defined periods between 2010–2014 and 2015–2017, in accordance with modification of the patient management protocol of the hospital (Fig. 1), based on the recommendations of the CPG of the AAP on the diagnosis, management and prevention of bronchiolitis.18 As modifications of the AAP guide, we included bronchodilator testing with salbutamol in patients over 6 months of age or with a predominance of wheezing, in order to limit its use to responders (decrease of ≥2 points on the severity scale); restriction of adrenaline spray use to the most severe cases (those admitted to the PICU) as rescue therapy; 3% hypertonic saline solution (HSS) as diluent in bronchodilator therapy or (on an isolated basis) in intensely secretory patients responding to no medication; and the indication of corticotherapy only in cases of post-extubation croup, croup prevention in patients subjected to intubation for over 5 days, and in infection due to rhinovirus. This guide was presented in a general session of the department and hospital, and was included on the institution intranet. Likewise, e-mails were sent to all the healthcare professionals to inform them about the mentioned changes.
Management algorithm corresponding to acute bronchiolitis in our hospital following publication of the Clinical Practice Guide (CPG) of the American Association of Pediatrics (AAP).
ABCDE: sequential evaluation; BROSJOD: Bronchiolitis Score of Sant Joan de Deu (bronchiolitis clinical severity scale); CPAP: continuous positive airway pressure; FiO2: fraction of inspired oxygen; HFO: high-flow oxygen therapy; PCO2: blood carbon dioxide partial pressure; Sat: hemoglobin saturation; 3% HSS: 3% hypertonic saline solution; PET: pediatric evaluation triangle.
Qualitative variables were expressed as frequencies and percentages, while quantitative variables were reported as the mean±standard deviation (SD) or as the median and interquartile range (IQR), as applicable. The comparison of qualitative variables was based on the chi-squared test, while quantitative variables were compared using the Student t-test or the Mann–Whitney U-test, depending on whether the data exhibited a normal distribution or otherwise. Statistical significance was considered for p<0.05. The SPSS® version 21.0 statistical package was used throughout.
Ethical considerationsThe study was approved by the Clinical Research Ethics Committee of the institution, and abided with the recommendations of the Declaration of Helsinki (latest edition, Fortaleza, Brazil, 2013).
ResultsDescriptive findingsThe study included a total of 706 patients with acute bronchiolitis (414 males; 58.6%). The median age was 47 days (IQR 25–100.25 days). A total of 66.57% of the patients were healthy nursing infants; prematurity was documented in 168 cases (23.79%), heart disease in 43 (6.09%), lung diseases in four (0.57%) and neurological disorders in 9 (1.27%). Of all these subjects, 5.38% (38 patients) had received immunoprophylaxis with palivizumab (30 patients with a history of prematurity and 8 patients with severe heart disease).
The PICU admission rate among the patients reporting to the emergency service and diagnosed with bronchiolitis was 7.74%. Most cases of bronchiolitis with admission to the PICU corresponded to community-acquired disease, while 5.66% (40 cases) were of nosocomial origin. Over one-half of the cases came from the hospital ward (386 cases, 54.67%). Patient origin is detailed in Table 1. The median severity score (BROSJOD) was 9 points (IQR 7–11), with a median PRISM-III score of 0 (IQR 0–3).
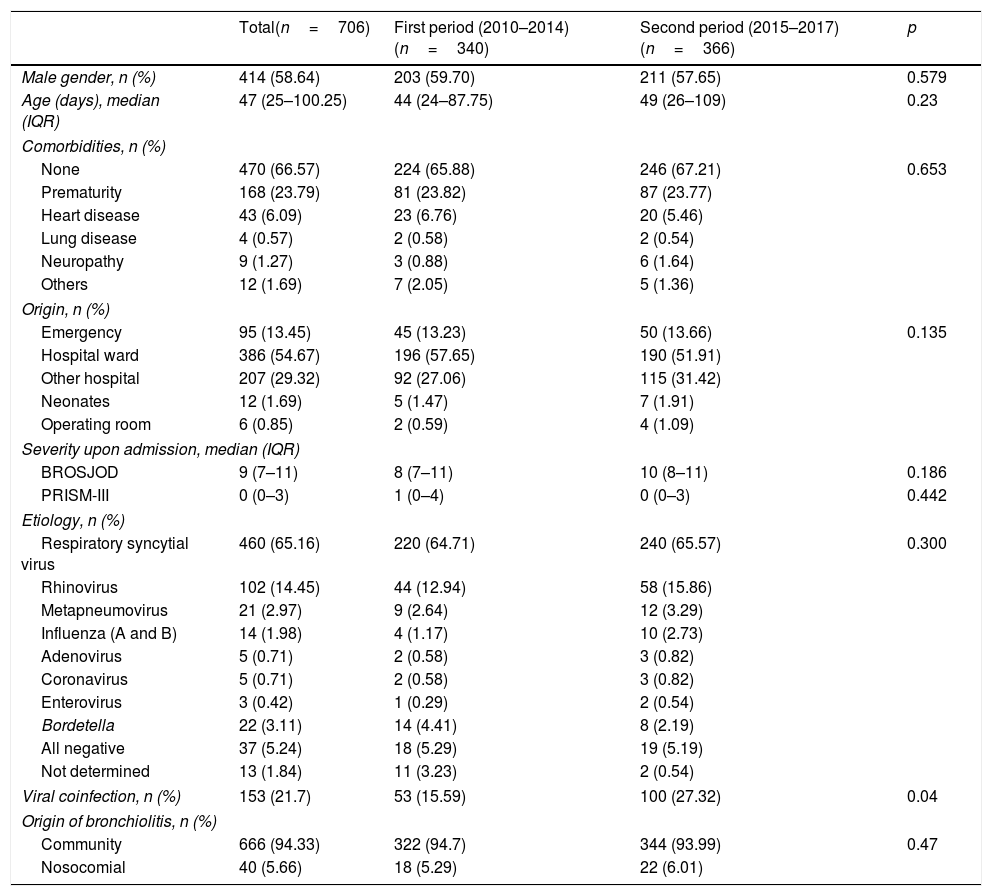
Epidemiological characteristics and etiology.
| Total(n=706) | First period (2010–2014)(n=340) | Second period (2015–2017)(n=366) | p | |
|---|---|---|---|---|
| Male gender, n (%) | 414 (58.64) | 203 (59.70) | 211 (57.65) | 0.579 |
| Age (days), median (IQR) | 47 (25–100.25) | 44 (24–87.75) | 49 (26–109) | 0.23 |
| Comorbidities, n (%) | ||||
| None | 470 (66.57) | 224 (65.88) | 246 (67.21) | 0.653 |
| Prematurity | 168 (23.79) | 81 (23.82) | 87 (23.77) | |
| Heart disease | 43 (6.09) | 23 (6.76) | 20 (5.46) | |
| Lung disease | 4 (0.57) | 2 (0.58) | 2 (0.54) | |
| Neuropathy | 9 (1.27) | 3 (0.88) | 6 (1.64) | |
| Others | 12 (1.69) | 7 (2.05) | 5 (1.36) | |
| Origin, n (%) | ||||
| Emergency | 95 (13.45) | 45 (13.23) | 50 (13.66) | 0.135 |
| Hospital ward | 386 (54.67) | 196 (57.65) | 190 (51.91) | |
| Other hospital | 207 (29.32) | 92 (27.06) | 115 (31.42) | |
| Neonates | 12 (1.69) | 5 (1.47) | 7 (1.91) | |
| Operating room | 6 (0.85) | 2 (0.59) | 4 (1.09) | |
| Severity upon admission, median (IQR) | ||||
| BROSJOD | 9 (7–11) | 8 (7–11) | 10 (8–11) | 0.186 |
| PRISM-III | 0 (0–3) | 1 (0–4) | 0 (0–3) | 0.442 |
| Etiology, n (%) | ||||
| Respiratory syncytial virus | 460 (65.16) | 220 (64.71) | 240 (65.57) | 0.300 |
| Rhinovirus | 102 (14.45) | 44 (12.94) | 58 (15.86) | |
| Metapneumovirus | 21 (2.97) | 9 (2.64) | 12 (3.29) | |
| Influenza (A and B) | 14 (1.98) | 4 (1.17) | 10 (2.73) | |
| Adenovirus | 5 (0.71) | 2 (0.58) | 3 (0.82) | |
| Coronavirus | 5 (0.71) | 2 (0.58) | 3 (0.82) | |
| Enterovirus | 3 (0.42) | 1 (0.29) | 2 (0.54) | |
| Bordetella | 22 (3.11) | 14 (4.41) | 8 (2.19) | |
| All negative | 37 (5.24) | 18 (5.29) | 19 (5.19) | |
| Not determined | 13 (1.84) | 11 (3.23) | 2 (0.54) | |
| Viral coinfection, n (%) | 153 (21.7) | 53 (15.59) | 100 (27.32) | 0.04 |
| Origin of bronchiolitis, n (%) | ||||
| Community | 666 (94.33) | 322 (94.7) | 344 (93.99) | 0.47 |
| Nosocomial | 40 (5.66) | 18 (5.29) | 22 (6.01) | |
BROSJOD: Bronchiolitis Score of Sant Joan de Deu (bronchiolitis clinical severity scale); IQR: interquartile range.
Respiratory syncytial virus was the cause of bronchiolitis in 460 patients (65.16%), followed by rhinovirus and metapneumovirus in 102 (14.45%) and 21 patients (2.97%), respectively. Viral coinfection was recorded in 21.7% of the cases, the most common combination being RSV and rhinovirus (40%), followed by RSV and coronavirus (12%). The etiological study is summarized in Table 1.
A total of 60.3% of the patients received treatment with inhaled 3% HSS. Other treatments received are described in Table 2. The post-extubation croup rate was 10.3%.
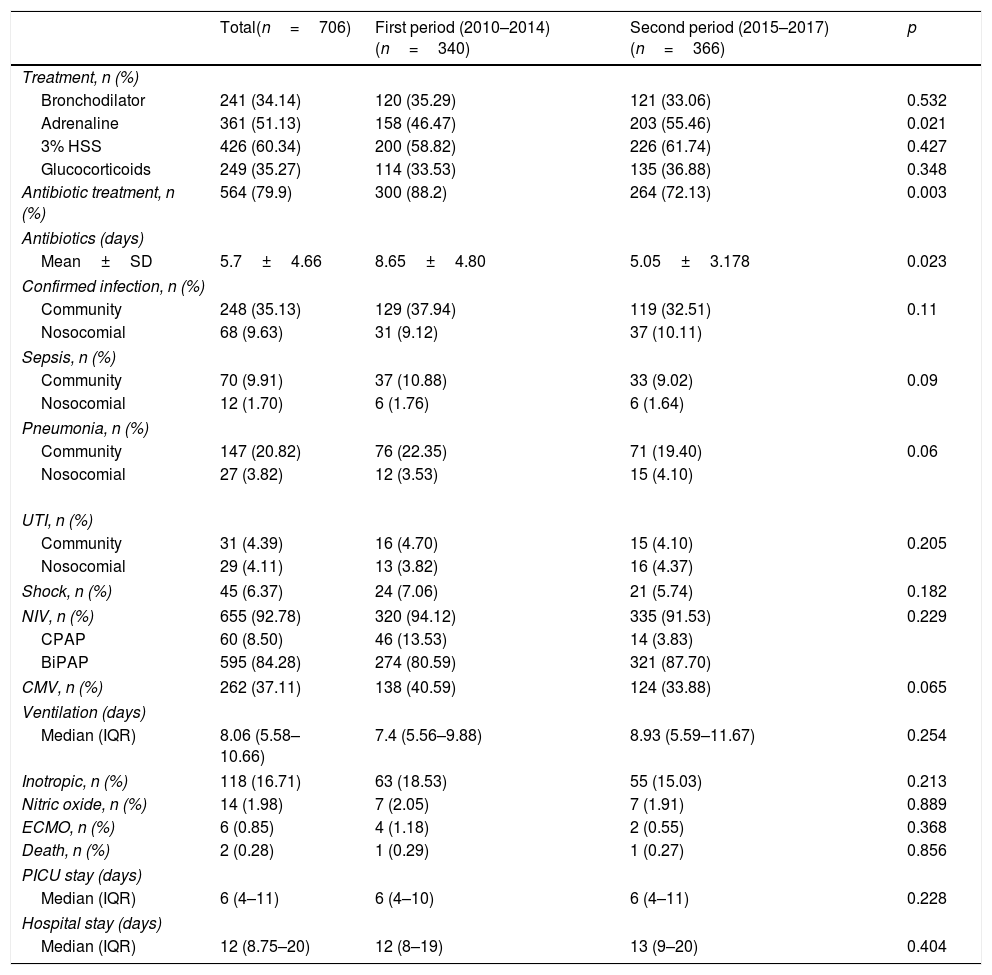
Treatment administered, bacterial infection and support measures adopted.
| Total(n=706) | First period (2010–2014)(n=340) | Second period (2015–2017)(n=366) | p | |
|---|---|---|---|---|
| Treatment, n (%) | ||||
| Bronchodilator | 241 (34.14) | 120 (35.29) | 121 (33.06) | 0.532 |
| Adrenaline | 361 (51.13) | 158 (46.47) | 203 (55.46) | 0.021 |
| 3% HSS | 426 (60.34) | 200 (58.82) | 226 (61.74) | 0.427 |
| Glucocorticoids | 249 (35.27) | 114 (33.53) | 135 (36.88) | 0.348 |
| Antibiotic treatment, n (%) | 564 (79.9) | 300 (88.2) | 264 (72.13) | 0.003 |
| Antibiotics (days) | ||||
| Mean±SD | 5.7±4.66 | 8.65±4.80 | 5.05±3.178 | 0.023 |
| Confirmed infection, n (%) | ||||
| Community | 248 (35.13) | 129 (37.94) | 119 (32.51) | 0.11 |
| Nosocomial | 68 (9.63) | 31 (9.12) | 37 (10.11) | |
| Sepsis, n (%) | ||||
| Community | 70 (9.91) | 37 (10.88) | 33 (9.02) | 0.09 |
| Nosocomial | 12 (1.70) | 6 (1.76) | 6 (1.64) | |
| Pneumonia, n (%) | ||||
| Community | 147 (20.82) | 76 (22.35) | 71 (19.40) | 0.06 |
| Nosocomial | 27 (3.82) | 12 (3.53) | 15 (4.10) | |
| UTI, n (%) | ||||
| Community | 31 (4.39) | 16 (4.70) | 15 (4.10) | 0.205 |
| Nosocomial | 29 (4.11) | 13 (3.82) | 16 (4.37) | |
| Shock, n (%) | 45 (6.37) | 24 (7.06) | 21 (5.74) | 0.182 |
| NIV, n (%) | 655 (92.78) | 320 (94.12) | 335 (91.53) | 0.229 |
| CPAP | 60 (8.50) | 46 (13.53) | 14 (3.83) | |
| BiPAP | 595 (84.28) | 274 (80.59) | 321 (87.70) | |
| CMV, n (%) | 262 (37.11) | 138 (40.59) | 124 (33.88) | 0.065 |
| Ventilation (days) | ||||
| Median (IQR) | 8.06 (5.58–10.66) | 7.4 (5.56–9.88) | 8.93 (5.59–11.67) | 0.254 |
| Inotropic, n (%) | 118 (16.71) | 63 (18.53) | 55 (15.03) | 0.213 |
| Nitric oxide, n (%) | 14 (1.98) | 7 (2.05) | 7 (1.91) | 0.889 |
| ECMO, n (%) | 6 (0.85) | 4 (1.18) | 2 (0.55) | 0.368 |
| Death, n (%) | 2 (0.28) | 1 (0.29) | 1 (0.27) | 0.856 |
| PICU stay (days) | ||||
| Median (IQR) | 6 (4–11) | 6 (4–10) | 6 (4–11) | 0.228 |
| Hospital stay (days) | ||||
| Median (IQR) | 12 (8.75–20) | 12 (8–19) | 13 (9–20) | 0.404 |
BiPAP: noninvasive bilevel positive airway pressure ventilation; CPAP: continuous positive airway pressure; SD: standard deviation; ECMO: extracorporeal membrane oxygenation; UTI: urinary tract infection; IQR: interquartile range; 3% HSS: 3% hypertonic saline solution; PICU: Pediatric Intensive Care Unit; CMV: conventional mechanical ventilation; NIV: noninvasive ventilation.
Empirical antibiotic therapy was started in 79.9% of the cases (564 patients) due to suspected infection, while in 13.6% of the cases such therapy was started due to confirmed infection. The mean global duration of antibiotic treatment was 5.7 days±4.66 days. Samples for blood culture were collected in 521 patients (74.2%) and for urine culture in 391 patients (55.3%), with lumbar puncture for CSF sampling being performed in 99 cases (12%). Bacterial infection was confirmed in 316 patients (44.7%): 82 corresponded to sepsis (11.6% of the total), 174 to pneumonia (24.6%) and 60 to urinary tract infection (8.5%). A total of 248 of the cases (35.13%) were considered to correspond to community-acquired bronchiolitis, while the rest were regarded as nosocomial presentations of the disease (68 patients; 9.63%) (Table 2).
Noninvasive ventilation (NIV) was required in 655 patients (92.78%): 60 with CPAP (8.50%) and 595 with BiPAP (84.28%). The main indication of NIV was breathing difficulty in 500 cases (78.5%), while in 65 patients (9.21%) the indication corresponded to clinically manifest apnea. Endotracheal intubation and conventional mechanical ventilation proved necessary in 262 patients (37.1%). The median total duration of ventilation was 8.06 days (IQR 5.58–10.66). The patients were admitted to the PICU for a median of 6 days (IQR 4–11 days). Inotropic support proved necessary in 118 patients (16.7%), with a mean duration of 1.16±0.4 days – the most frequently used drug being dopamine. Extracorporeal membrane oxygenation (ECMO) was required in 6 patients (0.85%). Lastly, two patients died due to bronchiolitis – one in each period. In one case death occurred secondary to concomitant type 2 herpes infection, while the other fatality was a consequence of bronchiolitis due to adenovirus with viral septic shock and progression to multiorgan failure (Table 2).
Comparative resultsThe first period involved 340 patients and the second 366 patients. On contrasting the two periods, no statistically significant differences were observed in terms of patient age, gender, the presence of comorbidities, origin or the etiology of bronchiolitis. In the second period, the BROSJOD severity score was seen to be higher (median of 10 versus 8 points), though the difference failed to reach statistical significance. Only in the case of viral coinfection was a significantly greater figure recorded in the second group (p=0.04). It therefore can be affirmed that the groups were quite homogeneous in comparative terms (Table 1).
The administration of inhaled salbutamol was similar in both periods. Inhaled adrenaline use was more prevalent in the second period (158 versus 203; p=0.021), in the same way as HSS (200 versus 226; p=0.427) and treatment with corticosteroids (114 versus 135; p=0.348) – though in the latter case statistical significance was not reached. Fewer patients were intubated in the second period (138 versus 124; p=0.065), though the latter was characterized by a greater number of patients with NIV (320 versus 336; p=0.229) (Table 2).
The indication of antibiotic treatment also decreased in the second period (72.13% versus 88.2% in the first period [p<0.05]). On comparing the two periods, the duration of antibiotic treatment was significantly shorter in the second period (p=0.023). The patients in the second period, who received antibiotic treatment for fewer days, experienced no clinical worsening and required no longer admission to the PICU.
Variables such as mortality, nitric oxide (NO) administration or extracorporeal membrane oxygenation, the duration of stay in the PICU and global hospital admission or inotropic drug support were similar in both groups (Table 2).
DiscussionAcute bronchiolitis is very common and remains a great challenge for healthcare professionals.
According to the reviewed literature, and in consistency with our own results, 2–3% of all patients with bronchiolitis require hospital admission, and of these, 3–11% must be admitted to the PICU.9,10
Respiratory syncytial virus remains the leading cause of bronchiolitis, as evidenced by all the published reviews, followed by rhinovirus and, at a distance, by other viruses such as metapneumovirus, influenza virus and coronavirus.3–5 This is consistent with our own observations. Viral coinfection is not a negligible phenomenon, and may imply a poorer course of the disease, with a tendency toward longer hospital stay.5–22
Following publication of the CPG of the AAP,18 we updated our protocol referred to the indication of nebulizations. Although the guide is very restrictive with inhaled therapy, the use of adrenaline or 3% HSS in the more serious cases is contemplated by the new guidelines. We maintained salbutamol in patients over 6 months of age that respond well to such treatment, even though it is not contemplated in the mentioned new guide.
The principal inhaled treatment in our study was 3% HSS. Its use is considered in the recommendations of the AAP in view of its probable mucociliary cleansing action, though only indirect supporting evidence of this is available. In contraposition, the recent article published by Zhang et al. does not appear to evidence effectiveness with the use of 3% HSS.23
Although inhaled adrenaline is not recommended on the grounds that it has not evidenced improvements in the outcome of patients with bronchiolitis, it is still administered in almost one-half of all patients, since it affords a degree of symptoms improvement,24 as was also observed in our own study. The increase in adrenaline use during the second time period cannot be attributed to increased patient severity, since the scores of the different scales did not differ significantly, though there was a tendency toward increased NIV use and a lesser application of invasive mechanical ventilation (albeit without statistically significant differences). One of our hypotheses is that more adrenaline was used due to the increased use of NIV (since inhaled adrenaline is not employed in intubated patients), though it cannot be discarded that administration of this drug rescues more patients that avoid the need for intubation. More extensive studies are needed in order to confirm this.
According to the literature, corticosteroid therapy would not be indicated,18 though it classically has been used in patients with spastic auscultation findings and over one year of age. In our protocol, corticosteroids were administered in patients with post-extubation croup (which represented 10.3% of the series) as croup prophylaxis from 5 days of mechanical ventilation25 and in those patients in which rhinovirus was isolated as the causal organism (90% of all rhinovirus cases in the second period were treated with corticosteroids), since it appears to be useful in some studies found in the literature.26–28 Despite modification of the protocol, we observed no decrease in corticosteroid use in patients diagnosed with bronchiolitis; its indication therefore again should be reconsidered in our Unit.
In any case, in view of the severity of patients with bronchiolitis requiring admission to the PICU, which moreover tend to be of younger age (generally under three months), and where intubation and mechanical ventilation imply a longer stay and an increased risk of complications, a reduction in treatment administration is a complicated issue. The emergency and primary care settings are probably the areas where increased adherence to the recommendations of the CPG of the AAP would be observed. In addition, it is important to remember the complex problem posed by the definitions used, which can result in classification and management difficulties.
The reported bacterial infection rate among patients with bronchiolitis requiring admission to the PICU is in the range of 40%, which is consistent with our own findings.29,30 The incidence drops to 3.5–12% in the cases analyzed in other settings such as emergency care or in hospital wards, and this consequently also usually implies a lesser use of antibiotics.31
The reason why antibiotic treatment in patients with acute respiratory failure remains high is concern about the possible presence of undetected bacterial infection.32 The antibiotic treatment rate in our series was high (79.9%), particularly considering that most of the patients were finally not diagnosed with bacterial infection. Nevertheless, the observed rate is similar to that reported in other studies conducted in the PICU setting.13,33 A recent study by Shein et al. justifies antibiotic treatment in the first two days of intubation among patients with severe bronchiolitis, on the grounds that it reduces the mean duration of stay.34
In our study, on comparing the two time periods, we observed a clear decrease in the indication of antibiotic treatment, together with a reduction in the duration of such treatment. This was probably due not only to the change in bronchiolitis management protocol in line with the recommendations of the AAP, but also to implementation of the antibiotic treatment optimization program in the PICU in the course of the second half of the year 2014. The adoption of these programs seeks to improve the clinical outcomes and ensure minimum toxicity, with a decrease in the development of resistances.35 In the case of severe bronchiolitis, the high antibiotic treatment rates observed both in our study and in the literature in general probably require the adoption of strategies for the early suspension of such treatment, as well as de-escalation measures. Accordingly, there is a clear need for diagnostic tools referred to invasive bacterial infection, since the signs and symptoms are very unspecific and difficult to distinguish from those of the viral condition.36 The use of biomarkers such as procalcitonin could help discriminate between the systemic inflammatory response generated by the viral infection and bacterial overinfection – thus allowing individualization of the indication and duration of antibiotic treatment.37 In our series, the patients in the second period that received antibiotic treatment during fewer days did not experience clinical worsening and did not require longer PICU stays; early de-escalation therefore seems to be a safe practice.
The single-center nature of our study constitutes a limitation in assessing the change in protocol following publication of the international recommendations. This study was designed to analyze the practice in our Unit and the points for future improvement. However, due to the large number of patients involved, the study allows acceptable comparison of the two patient samples in the two contemplated time periods.
ConclusionsBronchiolitis generates many admissions among nursing infants under one year of age. Knowing the epidemiological, clinical and microbiological data and their evolution over time has allowed us to analyze adherence to the international patient management guides and to identify weak and strong points in the protocols used at local level.
There are difficulties both for strictly applying the recommendations of the AAP in patients with more severe bronchiolitis and for modifying management protocols that are deeply rooted in traditional clinical practice. Revision of the indication of corticosteroid therapy and inhalatory treatment in severe bronchiolitis is probably needed.
AuthorshipDr. Carmina Guitart conducted initial data collection and analysis, drafted and reviewed the manuscript, and approved its final version.
Dr. Carme Alejandre conceived of and designed the study, conducted initial data collection and analysis, drafted and reviewed the manuscript, and approved its final version.
Dr. Isabel Torrus conducted data collection, drafted and reviewed the manuscript, and approved its final version.
Dr. Mònica Balaguer analyzed and interpreted the data, reviewed the manuscript, and approved its final version.
Dr. Elisabeth Esteban analyzed and interpreted the data, reviewed the manuscript, and approved its final version.
Dr. Francisco José Cambra analyzed and interpreted the data, reviewed the manuscript, and approved its final version.
Dr. Iolanda Jordan conceived of and designed the study, conducted initial data collection and analysis, performed the statistical analysis, drafted and reviewed the manuscript, and approved its final version.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Guitart C, Alejandre C, Torrús I, Balaguer M, Esteban E, Cambra FJ, et al. Impacto de una modificación de la guía de práctica clínica de la Academia Americana de Pediatría en el manejo de la bronquiolitis aguda grave en una unidad de cuidados intensivos pediátricos. Med Intensiva. 2021;45:289–297.