Sedation is a common procedure in intensive care (IC) that provides comfort and helps the patient to adapt to an environment often regarded as hostile. It is estimated that 2–30 % of all patients subjected to mechanical ventilation (MV) in IC experience suboptimum sedation phenomena, including difficult sedation.1,2
Difficult sedation is defined as “any situation in which a patient on MV needs larger than usual sedative doses to achieve the desired degree of sedation, or when problems arise derived from a decrease in the sedative dose being administered”.3 This situation is usually related to inadequate analgesia, tolerance to sedatives or hyperactive delirium, including privation – abstinence, and constitutes a challenge for management of the critically ill. A systematic and target-oriented approach may prove useful in this scenario.
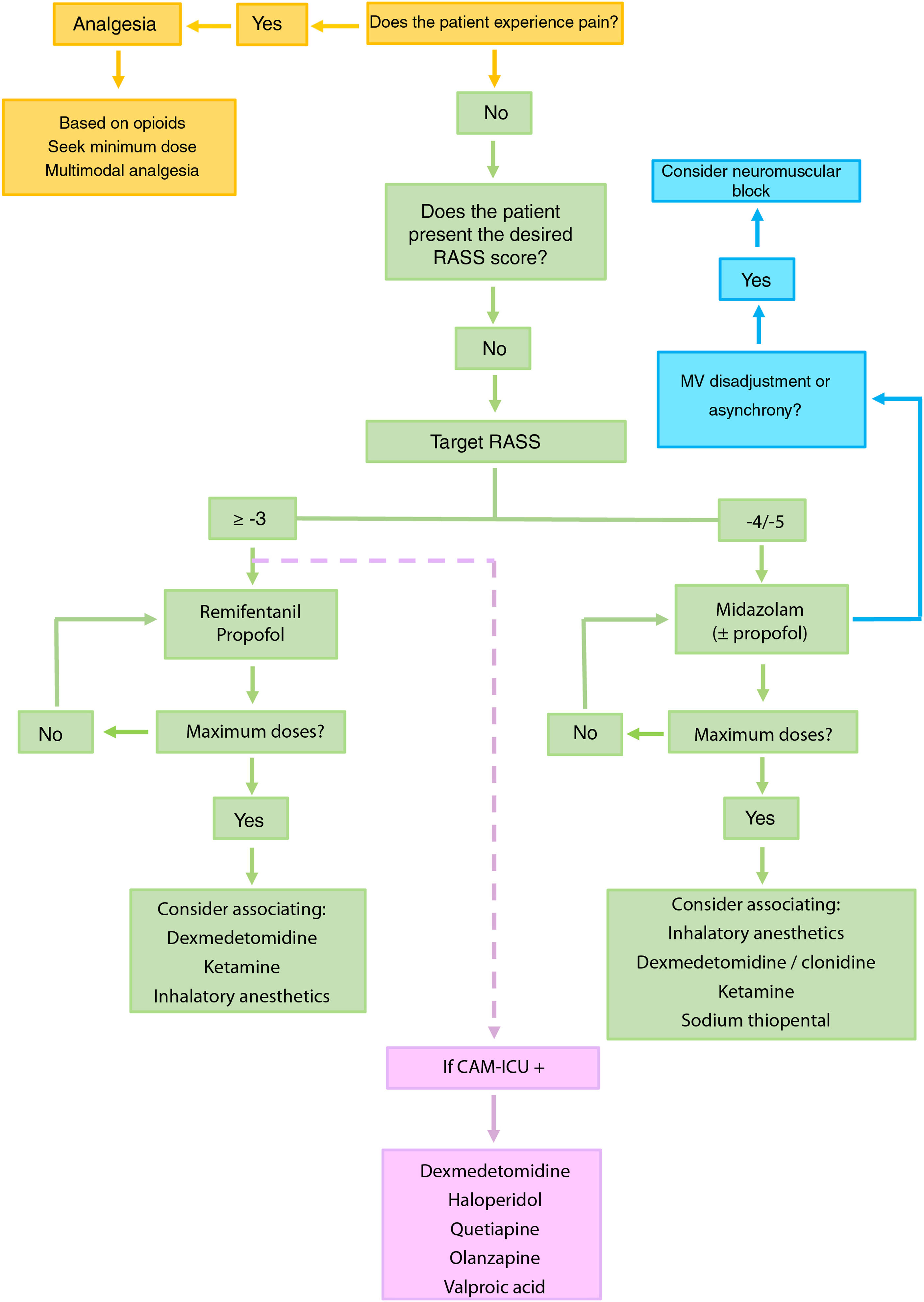
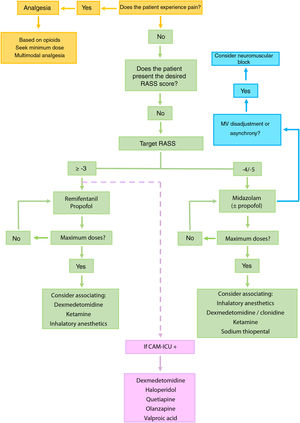
Proposed approach to patients with difficult sedationIdentification of pain and analgesiaThe identification of pain and the provision of good analgesia constitute the first step4–6 (Fig. 1).
Recommended algorithm for the management of patients with difficult sedation. The mentioned drugs are not stated according to order of priority. It is advisable to base the choice of drug taking into account the characteristics of the patient and the possible side effects.
Dexmedetomidine is recommended in sequential sedation (when we wish to switch from deep sedation to mild sedation), for the treatment of alcohol or pharmacological privation (including benzodiazepines), in patients subjected to extracorporeal membrane oxygenation (ECMO), and in situations of hyperactive delirium. Ketamine should be considered in patients with poor control of pain, status asthmaticus, in individuals subjected to ECMO or in hemodynamically unstable patients. In selected patients, inhalatory anesthetics may be regarded as a first choice, even over benzodiazepines, for maintaining RASS ≤ −4, and can also be used in patients with status asthmaticus or epilepticus. Although typical antipsychotics (haloperidol) and atypical antipsychotics (quetiapine and olanzapine) have not been shown to shorten the duration of delirium, MV or stay in IC, they can be used to control symptoms such as agitation, anxiety or hallucinations. Valproic acid can be used in the case of symptoms refractory to antipsychotic agents. In this regard, we can administer 1500 mg/day divided into 3–4 doses, that may be preceded by a loading dose of 28 mg/kg.
CAM-ICU: Confusion Assessment Method for the ICU.
Pain can be detected using self-administered scales (verbal numerical rating scale or visual analog scale [VAS]) in communicative patients, or using behavioral scales in non-communicative patients. In deep sedation (Richmond Agitation-Sedation Scale [RASS] score −5), where the validity of behavioral scales is questioned, we can use methods based on the variation of heart rate (Analgesia Nociception Index®), tools integrating different physiological parameters (Nociception Level Index®), or techniques that assess sympathetic response to harmful actions (video pupillometry).5
The basis of analgesia in IC is always an opioid.5,6 In the case of hyperacute pain requiring immediate control, remifentanil and fentanyl are the drugs of choice – both offering similar potency and onset of effect (1−3 min), with a short duration of action. The pharmacokinetic characteristics of remifentanil require continuous administration of the drug, while fentanyl can be used in isolated doses (50−150 μg) or in perfusion (30−100 μg/h).
Morphine, because of its lesser liposolubility, is characterized by a delay in its maximum effect (15−30 min); as a result, it is less appropriate for immediate pain control. Nevertheless, its longer half-life (up to 6 h) defines it as the ideal opiate for intermittent administration. Remifentanil is metabolized through plasma esterases; it is therefore the best choice opiate for patients with liver or kidney failure.
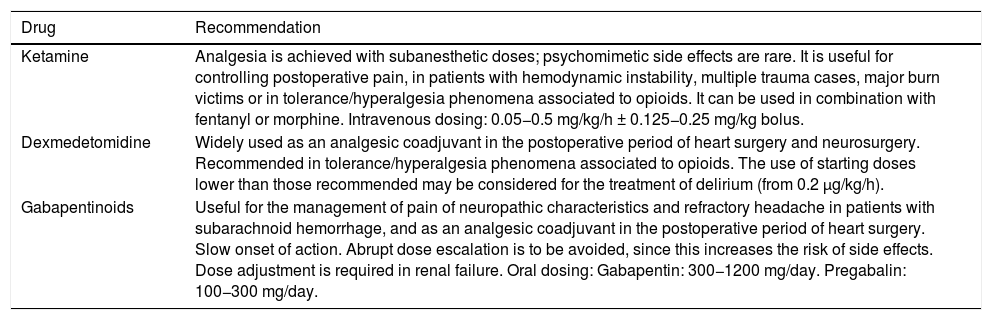
In some cases of difficult sedation, the use of multimodal analgesia comprising dexmedetomidine, ketamine or gabapentinoids, among other drugs, can help optimize pain control and reduce the total dose of opioids (Table 1).5,6
Drugs to be considered within a multimodal analgesia strategy.
| Drug | Recommendation |
|---|---|
| Ketamine | Analgesia is achieved with subanesthetic doses; psychomimetic side effects are rare. It is useful for controlling postoperative pain, in patients with hemodynamic instability, multiple trauma cases, major burn victims or in tolerance/hyperalgesia phenomena associated to opioids. It can be used in combination with fentanyl or morphine. Intravenous dosing: 0.05−0.5 mg/kg/h ± 0.125−0.25 mg/kg bolus. |
| Dexmedetomidine | Widely used as an analgesic coadjuvant in the postoperative period of heart surgery and neurosurgery. Recommended in tolerance/hyperalgesia phenomena associated to opioids. The use of starting doses lower than those recommended may be considered for the treatment of delirium (from 0.2 μg/kg/h). |
| Gabapentinoids | Useful for the management of pain of neuropathic characteristics and refractory headache in patients with subarachnoid hemorrhage, and as an analgesic coadjuvant in the postoperative period of heart surgery. Slow onset of action. Abrupt dose escalation is to be avoided, since this increases the risk of side effects. Dose adjustment is required in renal failure. Oral dosing: Gabapentin: 300−1200 mg/day. Pregabalin: 100−300 mg/day. |
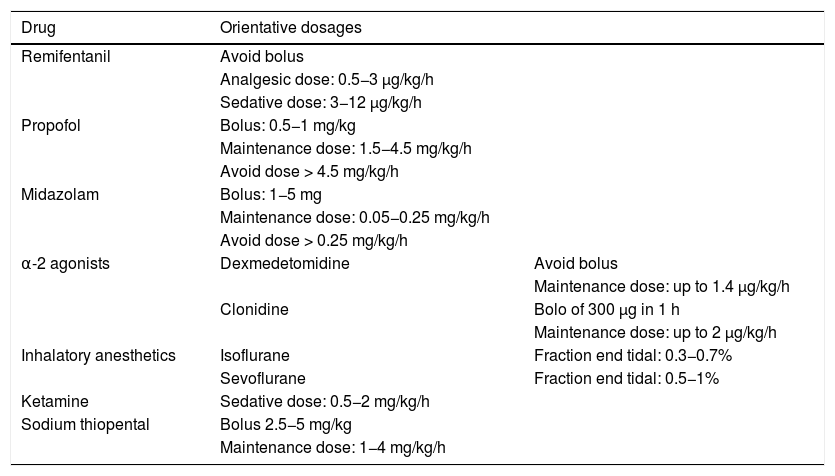
In order to avoid the development of tolerance to sedatives, it is advisable to define a daily sedation target,4–6 avoid the administration of doses higher than the recommended maximum dose (Table 2)3,7 and, in patients with RASS ≤ −4, manage MV asynchrony / disadaptation through point or continued neuromuscular block instead of an increase in sedation dose.8
Drugs for use in difficult sedation and their orientative dosages.
| Drug | Orientative dosages | |
|---|---|---|
| Remifentanil | Avoid bolus | |
| Analgesic dose: 0.5−3 μg/kg/h | ||
| Sedative dose: 3−12 μg/kg/h | ||
| Propofol | Bolus: 0.5−1 mg/kg | |
| Maintenance dose: 1.5−4.5 mg/kg/h | ||
| Avoid dose > 4.5 mg/kg/h | ||
| Midazolam | Bolus: 1−5 mg | |
| Maintenance dose: 0.05−0.25 mg/kg/h | ||
| Avoid dose > 0.25 mg/kg/h | ||
| α-2 agonists | Dexmedetomidine | Avoid bolus |
| Maintenance dose: up to 1.4 μg/kg/h | ||
| Clonidine | Bolo of 300 μg in 1 h | |
| Maintenance dose: up to 2 μg/kg/h | ||
| Inhalatory anesthetics | Isoflurane | Fraction end tidal: 0.3−0.7% |
| Sevoflurane | Fraction end tidal: 0.5−1% | |
| Ketamine | Sedative dose: 0.5−2 mg/kg/h | |
| Sodium thiopental | Bolus 2.5−5 mg/kg | |
| Maintenance dose: 1−4 mg/kg/h |
It is advisable to use the minimum drug dose needed to reach the established depth of sedation target.
The depth of sedation should be defined and monitored using scales such as the RASS. In the absence of contraindications, it is advisable for critical patients to maintain mild sedation (RASS score from 0 to −2), since this is associated to shorter times to extubation and a lesser incidence of tracheotomy, while deep sedation (RASS ≤ −4) can increase mortality, the incidence of delirium and the duration of MV.4–6
Propofol and remifentanil are characterized by rapid onset and cessation of effect, and do not accumulate in patients with liver or kidney failure; these properties define them as ideal options for mild sedation. However, in patients with severe hemodynamic instability or in those requiring deep sedation with or without neuromuscular block, we should consider the use of benzodiazepines (e.g., midazolam).4,7 In deep sedation, where the RASS would not detect oversedation, or in situations of neuromuscular block, where it is crucial to avoid undersedation, it is advisable to adjust sedation through objective monitoring (e.g., using the bispectral index [BIS]).5
When the maximum dose of sedative is unable to secure the RASS target score, we should consider a combination of drugs, since further incrementing the dose beyond the recommended maximum dose can produce important side effects. The “propofol infusion syndrome”, associated to doses of > 4.5 mg/kg/h, is a serious condition with an estimated mortality rate of over 80 %.9 In the case of the midazolam, although there is no direct correlation between dosage and mortality, it is advisable not to administer > 0.25 mg/kg/h, because doing so prolongs the duration of MV and increases the incidence of delirium and the duration of stay in IC.3,5
Apart from the possible combinations of opioids, propofol and midazolam, we can consider combinations with the following (Table 2):
- •
α-2 agonists: dexmedetomidine, rated above clonidine and at the same level as propofol, has emerged as one of the drugs to be considered among medical and postsurgical patients in IC that require mild sedation. This is due in part because compared with midazolam, dexmedetomidine appears to be associated with a lesser prevalence of delirium and a shorter duration of MV.5 The greater α-2 receptor affinity of dexmedetomidine, which results in a lesser incidence of cardiovascular adverse effects, as well as its intravenous availability, make this drug a more attractive option than clonidine, though no studies have demonstrated its superiority as a sedative.10
- •
Inhalatory anesthesia (isoflurane and sevoflurane): the excellent dose-response relationship, neuro- and cardioprotective properties, and the development of devices allowing its administration in the IC setting, define inhalatory anesthesia as a plausible alternative. These drugs are effective and safe, and in comparison with intravenous anesthetics, have demonstrated a shorter wake-up time and time to extubation.11
- •
Ketamine: at subanesthetic doses, ketamine has been shown to be a good analgesic adjuvant, but few studies have evaluated its role in the prolonged sedation of critical patients. Nevertheless, its side effects – including delirium – appear to be comparable to those of the rest of sedatives.12
- •
Sodium thiopental: this drug can be used as a last resort in cases of difficult sedation with target RASS ≤ −4. Its tendency to accumulate and its cardiodepressive action require careful dose titration using objective methods (electroencephalography or BIS).
The identification, prevention and treatment of delirium must form part of the routine in the critical patient.4–6
For the prevention and treatment of delirium, the use of non-pharmacological measures to improve patient cognitive capacity, sleep, vision, hearing or mobility can prove useful, although their application in IC remains low.2,5,6 In contrast, the use of mechanical restraints, with a prevalence of approximately 15% in Spain, has not been shown to reduce the risk of falls or patient self-removal of devices; the utilization of such measures is therefore not advised.2,5,13,14
In patients on MV with hyperactive delirium, dexmedetomidine is the drug of choice.15 Although the use of antipsychotics (typical drugs such as haloperidol or atypical drugs such as quetiapine, olanzapine, risperidone) or of other adjuvant agents such as valproic acid is not recommended on a routine basis, such drugs can be used for the treatment of positive symptoms (agitation, anxiety, aggressivity, etc.).5 If delirium is secondary to privation — abstinence (toxic substances or alcohol), the addition of a gabaergic drug together with an α-2 agonist may be the most useful strategy.
Adequate conciliation between the chronic medication of the patient and the medication received in IC is essential in order to avoid difficult sedation scenarios related to the abrupt suspension of agents such as benzodiazepines, antipsychotics, opioids and antidepressants, among others.
In conclusion, difficult sedation is a challenge in which a stepwise approach first centered on analgesia and then on target-guided dynamic sedation — both complemented by an adequate diagnosis and treatment of delirium - may constitute the best strategy for the management of these patients.
Financial supportThe present study has received no financial support.
Conflicts of interestThe authors declare that they have no conflicts of interest in relation to this publication.
Thanks are due to Drs. Carlos Chamorro and Miguel Angel Romera for their exhaustive review of the text and advice.
Please cite this article as: Alcántara Carmona S, García Sánchez M. Manejo del paciente con sedación difícil en el ámbito de la Medicina Intensiva. Med Intensiva. 2021;45:437–441.