A comparison was made of the oxidative stress (OS) levels of patients with either viral or bacterial severe community-acquired pneumonia (sCAP) and of patients without infection (healthy volunteers (HV) and patients with acute myocardial infarction (AMI)).
DesignA prospective observational study was made.
PatientsCritically ill patients with sCAP.
VariablesThe TBARS level was measured as an index of oxidative injury. SOD, CAT and redox glutathione system (GSH, GSSG, GR, GPx) activities were measured as reflecting antioxidant capacity. Severity of illness was assessed by the APACHE II, SOFA and SIRS scores.
ResultsThirty-seven subjects were included: 15 patients with CAP (12 of bacterial origin [BCAP] and 3 due to 2009 A/H1N1 virus [VCAP]), 10 HV and 12 AMI patients. Intensive care CAP mortality was 26.7% (n=4). Plasmatic TBARS levels were higher in CAP patients than in HV, but similar to those recorded in AMI patients. In contrast, VCAP was associated with lower TBARS levels, and some components of the glutathione redox system were higher in BCAP patients and HV. The OS levels did not differ between survivors and non-survivors.
ConclusionOur results suggest the occurrence of higher OS in sCAP patients compared with HV. In contrast, lower TBARS levels were observed in VCAP patients, suggesting an increase of antioxidant activity related to the redox glutathione system. However, further research involving a larger cohort is needed in order to confirm these findings.
Comparar el estrés oxidativo (EO) en pacientes con neumonía comunitaria grave (NCG) según su etiología y respecto de voluntarios sanos (VS) y pacientes con infarto agudo de miocardio (IAM).
DiseñoEstudio prospectivo, observacional.
PacientesPacientes con NCG ingresados en unidades de cuidados intensivos.
VariablesLos niveles de lipoperoxidación (TBARS) fueron considerados como índice de oxidación, mientras que SOD, CAT y la actividad del sistema redox- glutation (GSH, GSSG, GR, GPx) fueron considerados capacidad antioxidante. La gravedad de los pacientes fue valorada mediante las escalas APACHE II, SOFA y SIRS.
ResultadosTreinta y siete sujetos fueron incluidos, 15 pacientes con NCG (12 con etiología bacteriana [NB] y 3 viral 2009 A/H1N1 [NV]), 10 VS y 12 con IAM. La mortalidad global fue del 26,7% (n=4). Los TBARS plasmáticos fueron superiores en NCG respecto de VS, pero similares al IAM. En contraste, la NV se asoció con menores niveles de TBARS e incremento de componentes del sistema redox-glutation respecto de NB y voluntarios sanos. No se observó asociación entre mortalidad y EO.
ConclusiónNuestros resultados evidencian la presencia de EO en pacientes con NCG respecto de los controles. En contraste, la evidencia de un menor nivel de TBARS en la NV respecto de los VS sugiere un incremento de la actividad antioxidante relacionada con el sistema redox-glutation. Sin embargo, son necesarias nuevas investigaciones para confirmar estos hallazgos.
Community-acquired pneumonia (CAP) continues, even nowadays, to be one of the most common causes of admission in Intensive Care Units (ICU) with a significant morbidity. It is, therefore, not surprising that much research has been done in improving outcomes associated with this disease.
Reactive oxygen species (ROS) are constantly produced under physiological conditions and balanced between pro- and antioxidant activity.1 Nevertheless, in CAP, such activity might drastically be enhanced in response to primary host defence mechanism through phagocytes activation. Immune cell functions, activation of inflammatory cascades and expression of adhesion molecules are specially linked to ROS generation.2 Due to the respiratory burst generated by the massive flux of phagocytes, an important generation and release of ROS is produced.
The “respiratory burst” in a phagocyte is triggered when a bacteria is phagocytised. Therefore an important generation and release of ROS might perpetuate or increase the inflammatory response. Lipids, proteins and DNA damage oxidation leads to a tissue injury.3–5 In this context, the organism is overwhelmed by an imbalance between oxidant generation and antioxidant defenses; this situation is defined as oxidative stress (OS)6 and contributes to cellular derangement, cell injury and death.
Lipid peroxidation mediated by free radicals is considered to be the major mechanism of cell membrane destruction and cell damage.7 Some of the antioxidant systems that remove or inactivate ROS are superoxide dismutase (SOD; E.C. 1.15.1.1), catalase (CAT, E.C.1.11.1.6), glutathione redox system: reduced glutathione (GSH), glutathione disulfide (GSSG), glutathione reductase (GR), glutathione peroxidase (GPx, E.C. 1.11.1.9). Several studies8–10 have demonstrated that OS occurs in different settings affecting critically ill patients, such as in ARDS or organ dysfunction.
Only a few studies have focussed on analyzing the role of OS in the pathophysiology of bacterial pneumonia in humans.11–14 Moreover, most of them are based on experimental models. In addition, little is known regarding the pathogenesis of 2009 H1N1 viral pulmonary infection15,16 and ROS production.17,18 In fact, viral and bacterial pulmonary infection may play a different role in the pro- and antioxidant balance in host cells.19,20 Thus, our aim was to compare the oxidative stress in severely ill patients with community-acquired pneumonia according to aetiology and with respect to a group of healthy volunteers and patients who suffered an acute myocardial infarction.
Materials and methodsPopulation samplesThis study has been conducted according to the principles expressed in the Declaration of Helsinki and after obtaining local Ethics Committee approval. Signed informed consent was obtained from all patients or relatives and healthy volunteers. Oxidative stress data were not used in patients’ management and did not interfere with patient care.
Consecutive patients at ICU admission were included in a prospective and observational study with diagnosis of bacterial community-acquired pneumonia (BCAP) according to ATS/IDSA Guidelines21 or confirmed 2009 pandemic Influenza A/H1N1 pneumonia (VCAP) as reported elsewhere.22 In brief, BCAP was defined as an acute lower respiratory tract infection characterized by (a) an acute pulmonary infiltrate evident on chest radiography and consistent with pneumonia; (b) confirmatory findings of a clinical examination; (c) acquisition of the infection outside a hospital, long-term care facility, or nursing home23 associated with positive bacteriologic data or with a favorable outcome following antibiotic therapy.24 An organism was considered to be a “definite” etiologic agent only if it could be isolated from samples of blood or pleural fluid or if serological tests revealed a four-fold increase in antibody levels. Isolation of Pneumocystis jiroveci or culture of Legionella pneumophila or Mycobacterium tuberculosis from any of the samples was considered to be the basis of a “definite” diagnosis. Other microorganisms isolated from sputum, tracheal aspirate, protected specimen brush (PSB), or bronchoalveolar lavage (BAL) fluid samples were considered to be “probable” pathogens. A urinary antigen test result positive for Legionella pneumophila or Streptococcus pneumoniae was considered to provide a “probable” aetiology.25
The 2009 A/H1N1 primary viral pneumonia (VCAP) was defined in patients presenting during the acute phase of influenza virus illness with acute respiratory disease and unequivocal alveolar opacification involving two or more lobes with negative respiratory and blood bacterial cultures.22 The 2009 A/H1N1 infection was confirmed by means of real-time reverse-transcription-polymerase chain reaction (RT-PCR) on either nasopharyngeal swab samples or tracheal secretions (bronchoaspirate). RT-PCR methods and further details are described elsewhere.22,23 A confirmed case was defined as an acute respiratory illness with laboratory-confirmed An/H1N1 and included in the study.
Severe CAP was defined as patients with CAP requiring ICU admission. Patients with HIV infection, neoplasia, those taking cytotoxic drugs or long-term oral steroid therapy, such as daily dose of 20mg of prednisolone or the equivalent for >2 weeks were considered immune-compromised and were excluded.
Two groups were considered for comparison:
- a)
Control group (CG): Ten healthy volunteers were included; they were matched with patients according to age (±5 years).
- b)
Non-infectious group: Twelve patients with non-complicated acute myocardial infarction (AMI) were included. AMI was considered as a good group for comparison because it is an acute pathology of non-infectious origin, which induces a systemic inflammatory response with production of oxygen free radical and tissue damage. This non-infectious group of patients were matched by age (±5 years) and onset time of acute pathology (24h) with the study group patients. AMI was defined as a clinical (or pathologic) event caused by myocardial ischemia in which there is evidence of myocardial injury or necrosis.26,27 Criteria are met when there is a rise and/or fall of cardiac biomarkers, along with supportive evidence in the form of typical symptoms, suggestive electrocardiographic changes, or imaging evidence of new loss of viable myocardium or new regional wall motion abnormality.
In all patients, clinical, demographics and biochemical variables were recorded to determine severity of illness by measuring Acute Physiology and Chronic Health Evaluation (APACHE II score),28 Sequential Organ Failure Assessment (SOFA score)29 and Systemic Inflammatory Response Syndrome (SIRS score).30
Groups analysisSubjects were distributed into 3 groups: (1) CAP patients (study group), (2) healthy volunteers (control Group) and (3) AMI (non-infectious group) for comparison. Subsequently, we performed a comparison within the CAP group differentiating bacterial (BCAP) vs. 2009 A/H1N1 viral (VCAP) etiology. In addition, BCAP and VCAP patients were compared with healthy volunteers group.
Samples collectedAfter obtaining informs consent from the patient or her/his legal representative and healthy volunteers, blood samples were obtained from all patients on ICU admission, day 3 and ICU discharge. In healthy volunteers only one blood sample was collected.
Oxidative stress analysisThey were collected in lithium–heparin as well as EDTA tubes. The haemoglobin (Hb) concentration was determined by a spectrophotometer (Perkin Elmer Lambda 2) at 540nm. The blood was centrifuged at 2000rpm (850×g) for 15min to obtain plasmatic and erythrocytary fractions. Plasma was separated and stored at −80°C until analysis. The erythrocytes were washed twice with saline solution, processed and stored at −80°C until analysis. Index of oxidative injury (lipoperoxidative damage) was determined by MDA (Malondialdehyde). Antioxidant capacity was determined by copper–zinc superoxide dismutase (SOD), CAT and glutathione redox system (GSSG, GSH, GR, GPx) activities. GSSG/GSH ratio was calculated as redox buffer index.
Assay of plasma and erythrocytes MDAMDA concentration was estimated by measurement of thiobarbituric acid reactant substances (TBARS) by a modified fluorescence method31. Briefly, one volume of sample was mixed with 2vol of a solution of 15% (w/v) trichloroacetic acid, 0.375% (w/v) thiobarbituric acid and 0.25N hydrochloric acid, and the mixture heated for 15min in a boiling water bath. After cooling, the precipitate was removed by centrifugation at 3000rpm (1900×g) for 10min. Absorbance was measured at 548nm. Plasma and erythrocytes MDA are expressed as nmol/ml and nmol/g Hb, respectively.
Assays of enzymes activitiesSOD activity in erythrocytes was measured by the epinephrine auto-oxidation according to the method described by Mishra et al.32 One unit of SOD activity was defined as the amount of enzyme required to inhibit the rate of epinephrine auto-oxidation by 50%. The SOD activity results are expressed in UI/g Hb. CAT activity in erythrocytes was measured by the rate of decrease in the hydrogen peroxide (H2O2) absorbency at 240nm.33 Results of CAT activity are expressed in mmol/min/gHb. GPx activity in erythrocytes was determined by the decrease of NADPH at 340nm as described Wheeler CR et al.34. The result is expressed as μmol/min/g Hb. GR activity was determined by the increase of NADP+ at 340nm according to the method of Goldberg et al.35 GR activity is expressed in μmol/min/g Hb.
Assay of GSH and GSSGGSH and GSSG concentrations were determined according to Hissin et al.36 The reduced and oxidized thiols were determined by reaction with O-phtataldehyde for color development detected at 350 and 420nm by a spectrofluorimeter Perkin Elmer LS 50B. Plasma levels are expressed as nmol/ml and erythrocytes levels are expressed as μmol/g Hb.
Statistical analysisDiscrete variables were expressed as counts (%) and continuous variables as mean and standard deviation (SD), unless stated otherwise; all statistical tests were two-sided. p<0.05 was considered significant. Differences in categorical variables were calculated using two-sided likelihood ratio Chi-square test or Fisher exact test and the Mann–Whitney U test or Kruskal–Wallis test were used for continuous variables, when appropriate. To evaluate the predictive ability of the different biomarkers in regard with mortality, we calculated a receiver operator characteristic (ROC) curve and the area under the ROC (AUROC) curve. We calculate the AUROC because the area does not depend only on a particular portion of the plot, such as the point closest to the diagonal or the sensibility at 90%, but also on the entire plot, and provides a comprehensive picture of the ability of a test to make a distinction over all decision thresholds.
Data analysis was performed using SPSS for Windows 13.0.0 (SPSS, Chicago, IL, US).
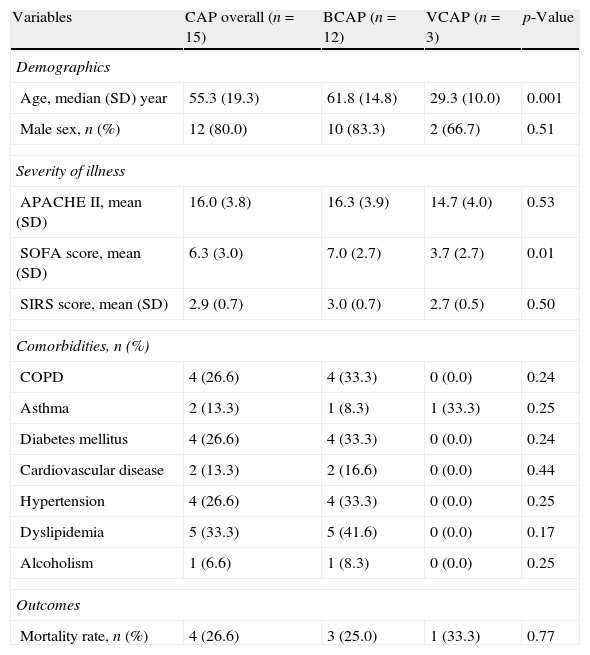
ResultsStudy groupFifteen patients with severe CAP were included and 450 measurements of OS biomarkers were performed. Among the study group, 12 (80%) patients had bacterial CAP (BCAP) and 3 patients (20%) had 2009 A/H1N1 viral pneumonia without bacterial co-infection (VCAP). Clinical characteristics of patients are shown in Table 1. BCAP patients were older and had higher level of organic dysfunction (SOFA score) than patients with VCAP. However, APACHE II score, SIRS score, comorbidities and mortality were not different between groups.
Clinical characteristics of 15 patients with community-acquired pneumonia (CAP) differentiating bacterial (BCAP) and viral (VCAP) etiology.
| Variables | CAP overall (n=15) | BCAP (n=12) | VCAP (n=3) | p-Value |
| Demographics | ||||
| Age, median (SD) year | 55.3 (19.3) | 61.8 (14.8) | 29.3 (10.0) | 0.001 |
| Male sex, n (%) | 12 (80.0) | 10 (83.3) | 2 (66.7) | 0.51 |
| Severity of illness | ||||
| APACHE II, mean (SD) | 16.0 (3.8) | 16.3 (3.9) | 14.7 (4.0) | 0.53 |
| SOFA score, mean (SD) | 6.3 (3.0) | 7.0 (2.7) | 3.7 (2.7) | 0.01 |
| SIRS score, mean (SD) | 2.9 (0.7) | 3.0 (0.7) | 2.7 (0.5) | 0.50 |
| Comorbidities, n (%) | ||||
| COPD | 4 (26.6) | 4 (33.3) | 0 (0.0) | 0.24 |
| Asthma | 2 (13.3) | 1 (8.3) | 1 (33.3) | 0.25 |
| Diabetes mellitus | 4 (26.6) | 4 (33.3) | 0 (0.0) | 0.24 |
| Cardiovascular disease | 2 (13.3) | 2 (16.6) | 0 (0.0) | 0.44 |
| Hypertension | 4 (26.6) | 4 (33.3) | 0 (0.0) | 0.25 |
| Dyslipidemia | 5 (33.3) | 5 (41.6) | 0 (0.0) | 0.17 |
| Alcoholism | 1 (6.6) | 1 (8.3) | 0 (0.0) | 0.25 |
| Outcomes | ||||
| Mortality rate, n (%) | 4 (26.6) | 3 (25.0) | 1 (33.3) | 0.77 |
SD: standard deviation; n: number of patients; APACHE II: acute physiology and chronic health evaluation; SOFA: sequential organ failure assessment; SIRS: systemic inflammatory response syndrome; COPD: chronic obstructive pulmonary disease.
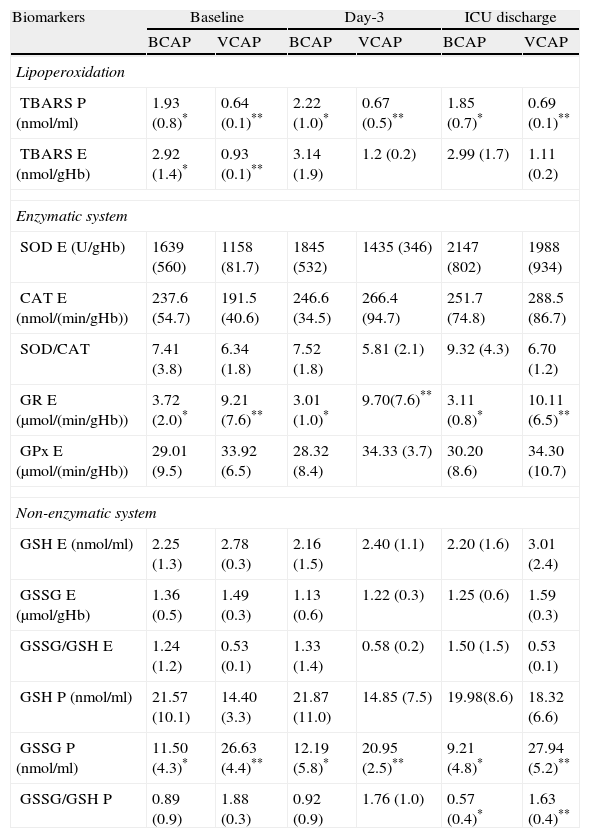
At baseline, day-3 and ICU discharge patients with VCAP showed lower plasmatic TBARS levels than patients with BCAP. Erythrocyte TBARS levels were also lower in patients with VCAP during entire study period. However, this difference only achieved significance at ICU admission (Table 2).
Oxidative stress biomarkers at ICU admission (baseline), day 3 and ICU discharge in patients with community-acquired pneumonia (CAP) differentiating bacterial (BCAP) and viral (VCAP) etiology.
| Biomarkers | Baseline | Day-3 | ICU discharge | |||
| BCAP | VCAP | BCAP | VCAP | BCAP | VCAP | |
| Lipoperoxidation | ||||||
| TBARS P (nmol/ml) | 1.93 (0.8)* | 0.64 (0.1)** | 2.22 (1.0)* | 0.67 (0.5)** | 1.85 (0.7)* | 0.69 (0.1)** |
| TBARS E (nmol/gHb) | 2.92 (1.4)* | 0.93 (0.1)** | 3.14 (1.9) | 1.2 (0.2) | 2.99 (1.7) | 1.11 (0.2) |
| Enzymatic system | ||||||
| SOD E (U/gHb) | 1639 (560) | 1158 (81.7) | 1845 (532) | 1435 (346) | 2147 (802) | 1988 (934) |
| CAT E (nmol/(min/gHb)) | 237.6 (54.7) | 191.5 (40.6) | 246.6 (34.5) | 266.4 (94.7) | 251.7 (74.8) | 288.5 (86.7) |
| SOD/CAT | 7.41 (3.8) | 6.34 (1.8) | 7.52 (1.8) | 5.81 (2.1) | 9.32 (4.3) | 6.70 (1.2) |
| GR E (μmol/(min/gHb)) | 3.72 (2.0)* | 9.21 (7.6)** | 3.01 (1.0)* | 9.70(7.6)** | 3.11 (0.8)* | 10.11 (6.5)** |
| GPx E (μmol/(min/gHb)) | 29.01 (9.5) | 33.92 (6.5) | 28.32 (8.4) | 34.33 (3.7) | 30.20 (8.6) | 34.30 (10.7) |
| Non-enzymatic system | ||||||
| GSH E (nmol/ml) | 2.25 (1.3) | 2.78 (0.3) | 2.16 (1.5) | 2.40 (1.1) | 2.20 (1.6) | 3.01 (2.4) |
| GSSG E (μmol/gHb) | 1.36 (0.5) | 1.49 (0.3) | 1.13 (0.6) | 1.22 (0.3) | 1.25 (0.6) | 1.59 (0.3) |
| GSSG/GSH E | 1.24 (1.2) | 0.53 (0.1) | 1.33 (1.4) | 0.58 (0.2) | 1.50 (1.5) | 0.53 (0.1) |
| GSH P (nmol/ml) | 21.57 (10.1) | 14.40 (3.3) | 21.87 (11.0) | 14.85 (7.5) | 19.98(8.6) | 18.32 (6.6) |
| GSSG P (nmol/ml) | 11.50 (4.3)* | 26.63 (4.4)** | 12.19 (5.8)* | 20.95 (2.5)** | 9.21 (4.8)* | 27.94 (5.2)** |
| GSSG/GSH P | 0.89 (0.9) | 1.88 (0.3) | 0.92 (0.9) | 1.76 (1.0) | 0.57 (0.4)* | 1.63 (0.4)** |
* vs. **p<0.05 for intergroup comparison.
Throughout the study period, erythrocyte GR activity was significantly higher in patients with VCAP in respect of BCAP patients. No other significant differences were observed when different biomarkers of the enzymatic system were compared (Table 2). Within the biomarkers of non-enzymatic system, only plasmatic GSSG levels were significantly higher in patients with VCAP compared to BCAP patients during the entire study period (Table 2). No other significant differences were observed between groups, except for plasmatic GSSG/GSH ratio at ICU discharge.
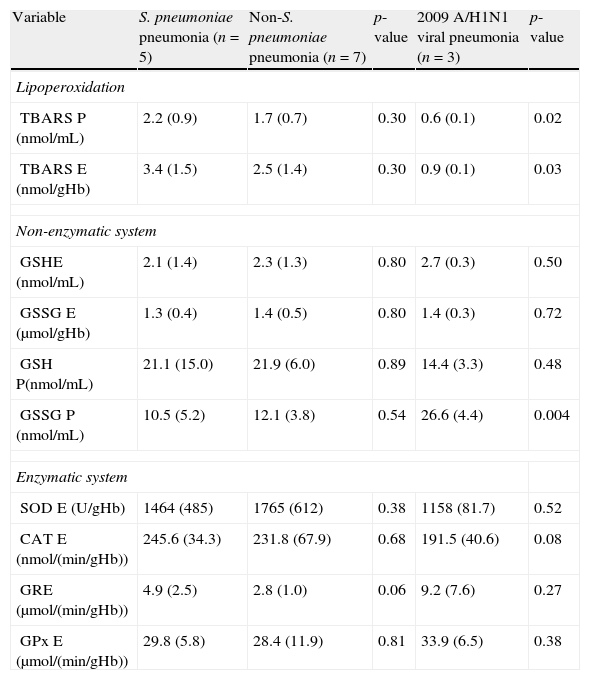
Microbiologic findings and oxidative stressEtiologic diagnosis was established in all cases of BCAP. A definite diagnosis was provided by blood culture in only 1 (8.3%) case. A probable diagnosis was provided by tests for urinary antigen detection in 4 cases (33.3%) and by bronchoscopic specimen or tracheal aspirate specimens with quantitative cultures in 8 (66.6%) patients. No diagnosis was made by sputum specimen culture. The most frequent microorganism isolated in BCAP was Streptococcus pneumoniae (n=5; 33.3%) followed by Legionella pneumophila (n=3; 20%), Staphylococcus aureus (n=2; 13.3%), Enterococcus faecalis (n=1; 6.7%) and Proteus mirabilis (n=1; 6.7%). No significant differences were observed when comparing oxidative stress biomarkers between pneumococcal and non-pneumococcal pneumonia. However, 2009 A/H1N1 viral aetiology was associated with lower levels of lipoperoxidation (Table 3).
Oxidative stress according to community-acquired pneumonia etiology.
| Variable | S. pneumoniae pneumonia (n=5) | Non-S. pneumoniae pneumonia (n=7) | p-value | 2009 A/H1N1 viral pneumonia (n=3) | p-value |
| Lipoperoxidation | |||||
| TBARS P (nmol/mL) | 2.2 (0.9) | 1.7 (0.7) | 0.30 | 0.6 (0.1) | 0.02 |
| TBARS E (nmol/gHb) | 3.4 (1.5) | 2.5 (1.4) | 0.30 | 0.9 (0.1) | 0.03 |
| Non-enzymatic system | |||||
| GSHE (nmol/mL) | 2.1 (1.4) | 2.3 (1.3) | 0.80 | 2.7 (0.3) | 0.50 |
| GSSG E (μmol/gHb) | 1.3 (0.4) | 1.4 (0.5) | 0.80 | 1.4 (0.3) | 0.72 |
| GSH P(nmol/mL) | 21.1 (15.0) | 21.9 (6.0) | 0.89 | 14.4 (3.3) | 0.48 |
| GSSG P (nmol/mL) | 10.5 (5.2) | 12.1 (3.8) | 0.54 | 26.6 (4.4) | 0.004 |
| Enzymatic system | |||||
| SOD E (U/gHb) | 1464 (485) | 1765 (612) | 0.38 | 1158 (81.7) | 0.52 |
| CAT E (nmol/(min/gHb)) | 245.6 (34.3) | 231.8 (67.9) | 0.68 | 191.5 (40.6) | 0.08 |
| GRE (μmol/(min/gHb)) | 4.9 (2.5) | 2.8 (1.0) | 0.06 | 9.2 (7.6) | 0.27 |
| GPx E (μmol/(min/gHb)) | 29.8 (5.8) | 28.4 (11.9) | 0.81 | 33.9 (6.5) | 0.38 |
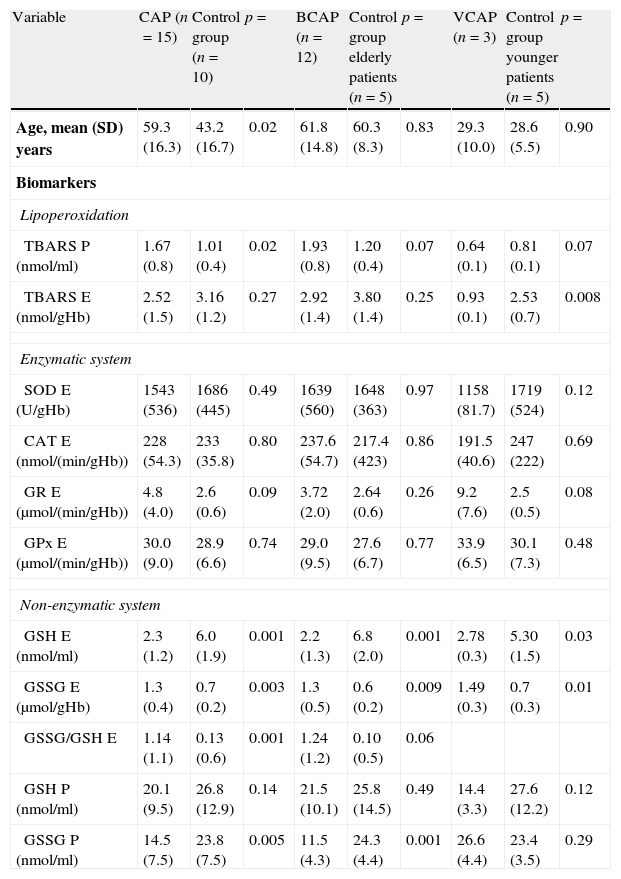
Ten healthy volunteers formed the control group. Plasmatic but not erythrocyte TBARS were higher in CAP patients. No differences were observed within enzymatic system between groups. Among non-enzymatic system, erythrocyte GSSG and GSSG/GSH ratio were higher in CAP patients. However, erythrocyte GSH and plasmatic GSSG were higher in HV (Table 4). CAP patients were older than control group patients due to the influence of patients with BCAP. This difference may explain the higher level of plasmatic TBARS observed. To avoid this possible confounding factor, we matched control group subjects with patients according to age. Two age-matched groups of healthy volunteers were compared with BCAP and VCAP patients respectively. Surprisingly, VCAP patients showed a lower level of lipoperoxidation with respect to controls. Significant differences were not observed when considering patients with BCAP (Table 4). Among enzymatic system, no significant differences were observed in all comparisons. However, GRE activity was higher in VCAP patients than control patients, although the difference was near to the significance (p=0.08). Lower levels of erythrocyte GSH and higher activity of GSSG E were observed in both BCAP and VCAP patients than in control group. In contrast, only BCAP patients showed lower levels of plasmatic GSSG than in control patients (Table 4).
Oxidative stress comparison between patients with community-acquired pneumonia (CAP) and control group of healthy volunteers in general and then matched by age.
| Variable | CAP (n=15) | Control group (n=10) | p= | BCAP (n=12) | Control group elderly patients (n=5) | p= | VCAP (n=3) | Control group younger patients (n=5) | p= |
| Age, mean (SD) years | 59.3 (16.3) | 43.2 (16.7) | 0.02 | 61.8 (14.8) | 60.3 (8.3) | 0.83 | 29.3 (10.0) | 28.6 (5.5) | 0.90 |
| Biomarkers | |||||||||
| Lipoperoxidation | |||||||||
| TBARS P (nmol/ml) | 1.67 (0.8) | 1.01 (0.4) | 0.02 | 1.93 (0.8) | 1.20 (0.4) | 0.07 | 0.64 (0.1) | 0.81 (0.1) | 0.07 |
| TBARS E (nmol/gHb) | 2.52 (1.5) | 3.16 (1.2) | 0.27 | 2.92 (1.4) | 3.80 (1.4) | 0.25 | 0.93 (0.1) | 2.53 (0.7) | 0.008 |
| Enzymatic system | |||||||||
| SOD E (U/gHb) | 1543 (536) | 1686 (445) | 0.49 | 1639 (560) | 1648 (363) | 0.97 | 1158 (81.7) | 1719 (524) | 0.12 |
| CAT E (nmol/(min/gHb)) | 228 (54.3) | 233 (35.8) | 0.80 | 237.6 (54.7) | 217.4 (423) | 0.86 | 191.5 (40.6) | 247 (222) | 0.69 |
| GR E (μmol/(min/gHb)) | 4.8 (4.0) | 2.6 (0.6) | 0.09 | 3.72 (2.0) | 2.64 (0.6) | 0.26 | 9.2 (7.6) | 2.5 (0.5) | 0.08 |
| GPx E (μmol/(min/gHb)) | 30.0 (9.0) | 28.9 (6.6) | 0.74 | 29.0 (9.5) | 27.6 (6.7) | 0.77 | 33.9 (6.5) | 30.1 (7.3) | 0.48 |
| Non-enzymatic system | |||||||||
| GSH E (nmol/ml) | 2.3 (1.2) | 6.0 (1.9) | 0.001 | 2.2 (1.3) | 6.8 (2.0) | 0.001 | 2.78 (0.3) | 5.30 (1.5) | 0.03 |
| GSSG E (μmol/gHb) | 1.3 (0.4) | 0.7 (0.2) | 0.003 | 1.3 (0.5) | 0.6 (0.2) | 0.009 | 1.49 (0.3) | 0.7 (0.3) | 0.01 |
| GSSG/GSH E | 1.14 (1.1) | 0.13 (0.6) | 0.001 | 1.24 (1.2) | 0.10 (0.5) | 0.06 | |||
| GSH P (nmol/ml) | 20.1 (9.5) | 26.8 (12.9) | 0.14 | 21.5 (10.1) | 25.8 (14.5) | 0.49 | 14.4 (3.3) | 27.6 (12.2) | 0.12 |
| GSSG P (nmol/ml) | 14.5 (7.5) | 23.8 (7.5) | 0.005 | 11.5 (4.3) | 24.3 (4.4) | 0.001 | 26.6 (4.4) | 23.4 (3.5) | 0.29 |
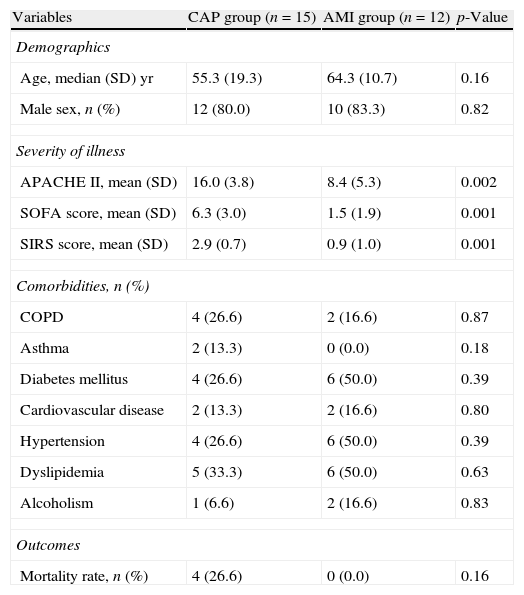
Twelve patients with acute myocardial infarction (AMI) were included in non-infectious patients group. Clinical characteristics of study and AMI group are shown in Table 5. Age, sex and comorbid conditions were not different between groups. However, as expected, the severity of illness measured by the APACHEII, SOFA and SIRS scores was higher in patients with CAP.
Clinical characteristics of patients with community-acquired pneumonia (CAP) and acute myocardial infarction (AMI).
| Variables | CAP group (n=15) | AMI group (n=12) | p-Value |
| Demographics | |||
| Age, median (SD) yr | 55.3 (19.3) | 64.3 (10.7) | 0.16 |
| Male sex, n (%) | 12 (80.0) | 10 (83.3) | 0.82 |
| Severity of illness | |||
| APACHE II, mean (SD) | 16.0 (3.8) | 8.4 (5.3) | 0.002 |
| SOFA score, mean (SD) | 6.3 (3.0) | 1.5 (1.9) | 0.001 |
| SIRS score, mean (SD) | 2.9 (0.7) | 0.9 (1.0) | 0.001 |
| Comorbidities, n (%) | |||
| COPD | 4 (26.6) | 2 (16.6) | 0.87 |
| Asthma | 2 (13.3) | 0 (0.0) | 0.18 |
| Diabetes mellitus | 4 (26.6) | 6 (50.0) | 0.39 |
| Cardiovascular disease | 2 (13.3) | 2 (16.6) | 0.80 |
| Hypertension | 4 (26.6) | 6 (50.0) | 0.39 |
| Dyslipidemia | 5 (33.3) | 6 (50.0) | 0.63 |
| Alcoholism | 1 (6.6) | 2 (16.6) | 0.83 |
| Outcomes | |||
| Mortality rate, n (%) | 4 (26.6) | 0 (0.0) | 0.16 |
SD: standard deviation; n: number of patients; APACHE II: acute physiology and chronic health evaluation; SOFA: sequential organ failure assessment; SIRS: systemic inflammatory response syndrome; COPD: chronic obstructive pulmonary disease.
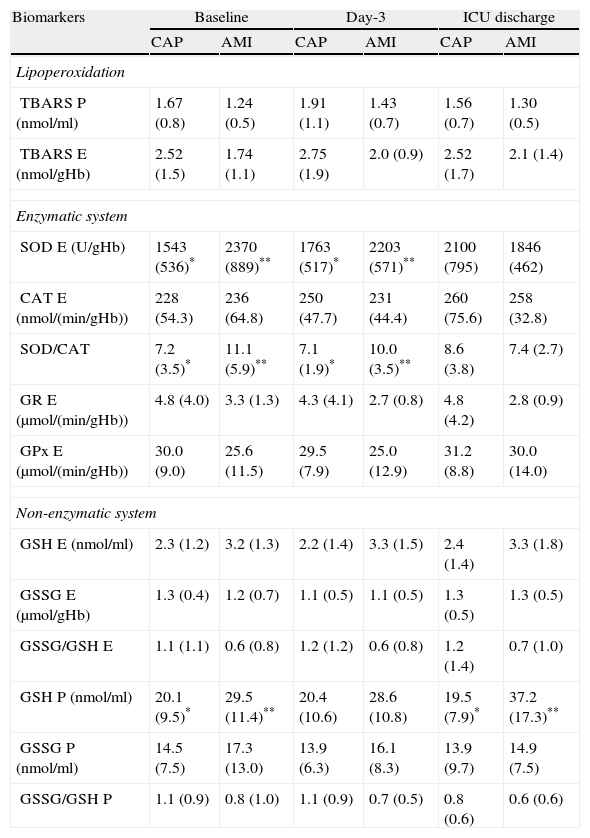
Although patients with CAP had higher levels of plasmatic and erythrocyte TBARS than patients with AMI, this difference did not reach statistical significance in any of the moments of the study (Table 6).
Oxidative stress biomarkers at ICU admission (baseline), day 3 and ICU discharge in the total population differentiating the study group (community-acquired pneumonia=CAP) and non-septic group (acute myocardial infarction=AMI).
| Biomarkers | Baseline | Day-3 | ICU discharge | |||
| CAP | AMI | CAP | AMI | CAP | AMI | |
| Lipoperoxidation | ||||||
| TBARS P (nmol/ml) | 1.67 (0.8) | 1.24 (0.5) | 1.91 (1.1) | 1.43 (0.7) | 1.56 (0.7) | 1.30 (0.5) |
| TBARS E (nmol/gHb) | 2.52 (1.5) | 1.74 (1.1) | 2.75 (1.9) | 2.0 (0.9) | 2.52 (1.7) | 2.1 (1.4) |
| Enzymatic system | ||||||
| SOD E (U/gHb) | 1543 (536)* | 2370 (889)** | 1763 (517)* | 2203 (571)** | 2100 (795) | 1846 (462) |
| CAT E (nmol/(min/gHb)) | 228 (54.3) | 236 (64.8) | 250 (47.7) | 231 (44.4) | 260 (75.6) | 258 (32.8) |
| SOD/CAT | 7.2 (3.5)* | 11.1 (5.9)** | 7.1 (1.9)* | 10.0 (3.5)** | 8.6 (3.8) | 7.4 (2.7) |
| GR E (μmol/(min/gHb)) | 4.8 (4.0) | 3.3 (1.3) | 4.3 (4.1) | 2.7 (0.8) | 4.8 (4.2) | 2.8 (0.9) |
| GPx E (μmol/(min/gHb)) | 30.0 (9.0) | 25.6 (11.5) | 29.5 (7.9) | 25.0 (12.9) | 31.2 (8.8) | 30.0 (14.0) |
| Non-enzymatic system | ||||||
| GSH E (nmol/ml) | 2.3 (1.2) | 3.2 (1.3) | 2.2 (1.4) | 3.3 (1.5) | 2.4 (1.4) | 3.3 (1.8) |
| GSSG E (μmol/gHb) | 1.3 (0.4) | 1.2 (0.7) | 1.1 (0.5) | 1.1 (0.5) | 1.3 (0.5) | 1.3 (0.5) |
| GSSG/GSH E | 1.1 (1.1) | 0.6 (0.8) | 1.2 (1.2) | 0.6 (0.8) | 1.2 (1.4) | 0.7 (1.0) |
| GSH P (nmol/ml) | 20.1 (9.5)* | 29.5 (11.4)** | 20.4 (10.6) | 28.6 (10.8) | 19.5 (7.9)* | 37.2 (17.3)** |
| GSSG P (nmol/ml) | 14.5 (7.5) | 17.3 (13.0) | 13.9 (6.3) | 16.1 (8.3) | 13.9 (9.7) | 14.9 (7.5) |
| GSSG/GSH P | 1.1 (0.9) | 0.8 (1.0) | 1.1 (0.9) | 0.7 (0.5) | 0.8 (0.6) | 0.6 (0.6) |
* vs. **p<0.05 for intergroup comparison/data reported as mean and standard deviation.
At baseline and day-3, erythrocyte SOD activity and SOD/CAT ratio were lower in CAP patients than in AMI patients. No significant differences were observed in other biomarkers of enzymatic system analyzed in different moments of the study. Within the biomarkers of non-enzymatic system, only the plasmatic GSH levels at ICU admission and ICU discharge were significantly lower in patients with CAP than in AMI patients (Table 6).
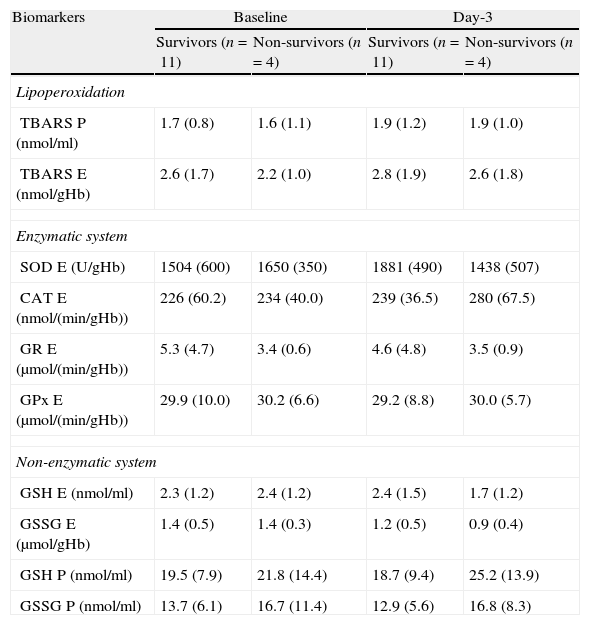
MortalityICU mortality of CAP was 26.6% (4/15). Three (25%) BCAP patients and 1 (33.3%) VCAP patient died. We found no significant differences in oxidative stress biomarkers between survivors and non-survivors at baseline and day-3 of study period (Table 7).
Comparison of oxidative stress biomarkers in survivors and non-survivors at baseline and day-3 of study period.
| Biomarkers | Baseline | Day-3 | ||
| Survivors (n=11) | Non-survivors (n=4) | Survivors (n=11) | Non-survivors (n=4) | |
| Lipoperoxidation | ||||
| TBARS P (nmol/ml) | 1.7 (0.8) | 1.6 (1.1) | 1.9 (1.2) | 1.9 (1.0) |
| TBARS E (nmol/gHb) | 2.6 (1.7) | 2.2 (1.0) | 2.8 (1.9) | 2.6 (1.8) |
| Enzymatic system | ||||
| SOD E (U/gHb) | 1504 (600) | 1650 (350) | 1881 (490) | 1438 (507) |
| CAT E (nmol/(min/gHb)) | 226 (60.2) | 234 (40.0) | 239 (36.5) | 280 (67.5) |
| GR E (μmol/(min/gHb)) | 5.3 (4.7) | 3.4 (0.6) | 4.6 (4.8) | 3.5 (0.9) |
| GPx E (μmol/(min/gHb)) | 29.9 (10.0) | 30.2 (6.6) | 29.2 (8.8) | 30.0 (5.7) |
| Non-enzymatic system | ||||
| GSH E (nmol/ml) | 2.3 (1.2) | 2.4 (1.2) | 2.4 (1.5) | 1.7 (1.2) |
| GSSG E (μmol/gHb) | 1.4 (0.5) | 1.4 (0.3) | 1.2 (0.5) | 0.9 (0.4) |
| GSH P (nmol/ml) | 19.5 (7.9) | 21.8 (14.4) | 18.7 (9.4) | 25.2 (13.9) |
| GSSG P (nmol/ml) | 13.7 (6.1) | 16.7 (11.4) | 12.9 (5.6) | 16.8 (8.3) |
p=no significant for all comparison.
Discrimination for mortality of different biomarkers and severity of illness scores at baseline was assessed using ROC. The area AUROC showed no consistent mortality discrimination for plasmatic TBARS (AUROC=0.32), GSH (AUROC=0.42), GSSG (AUROC=0.52), erythrocyte TBARS (AUROC=0.44), GSH (AUROC=0.58), GSSG (AUROC=0.42), CAT E (AUROC=0.52); GRE (AUROC=0.36), GPX (AUROC=0.44) and SOFA score (AUROC=0.68). Only SOD E (AUROC=0.72; 95%CI 0.46–0.98) and APACHE II score (AUROC=0.90; 95%CI 0.40–1.0) showed adequate mortality discrimination.
DiscussionThe current pilot study is the first to compare temporal patterns of oxidative stress in bacterial and 2009 A/H1N1 viral CAP and their relationship with respect to healthy volunteers and non-infectious patients group. Our findings have shown an increased OS and high TBARS levels in bacterial CAP compared with healthy volunteers but not with patients suffering an AMI. However and surprisingly, our results suggest that 2009 A/H1N1 viral CAP led to an antioxidant environment evidenced by lower TBARS levels and increased some component of the glutathione redox system when compared with healthy subjects, bacterial CAP and non-infectious patients.
Although ROS are important in the successful resolution of certain inflammatory states, such as infections, an imbalance in the redox homeostasis can result in excessive tissue injury and death cell. During CAP, an early response of the immune system against the infection comprises the migration of neutrophils into the infected tissue.37 In vivo, S. aureus infection is associated with enhance nitrate generation, myeloperoxidase (MPO) activity, lipoperoxidation levels, Protein carbonil levels and decrease GSH levels, and as well as decreased enzymatic antioxidant (SOD, CAT, GPx and GRE) activity.38 The GSH redox system is crucial in maintaining intracellular GSSG/GSH homeostasis, which is critical to normal cellular physiological processes, and represents one of the most important antioxidant defense systems in lung cells.39 In our study, CAP was associated with high levels of TBARS and a significant decrease of GSH levels when compared to healthy volunteers. This was accompanied with higher levels of GSSG and GSSG/GSH ratio. The maintenance of a low intracellular GSSG/GSH ratio minimizes accumulation of disulphide and provides a reducing environment within the cell. However, if any situation alters this ratio, this shift in the GSSG/GSH redox buffer influences a variety of cellular signaling processes, such as activation of the transcription factors AP-1 and NF-kB.40 Thus, depletion of intracellular GSH levels or increased GSSG levels is present concomitant with the induction of inflammatory mediators and chemotactic cytokines. This suggests that the intracellular redox state (GSSG/GSH levels) of the cell may play a role in the regulation and potentiation of the inflammatory responses in lung cells.
Myocardial ischemia or ischemia/reperfusion not only restores the blood supply but also causes massive productions of free radicals, resulting in an imbalance between the oxidative and anti-oxidative balance and may initiate lipid peroxidation in cell membranes. This situation can explain why we did not observe differences in TBARS levels between CAP and AMI patients. Our findings are similar to those of the study conducted by Dubois Rande et al.41 and Mac Murray et al.42 which reported a significant rise in TBARS levels with a concomitant decrease in antioxidant in patients with unstable angina and chronic heart failure.
Despite the limited sample size, it important to stress that the TBARS levels were lower in the VCAP with respect to the BCAP, AMI and healthy volunteers respectively. The impact of OS in BCAP has been extensively proved, however the role of blood antioxidants according to CAP etiology has not been totally elucidated. Our results suggest that an antioxidant status might play an important role in patients with 2009 A/H1N1 viral pneumonia. GR, GSSG, GSSG/GSH and GPx levels were more elevated in patients with viral pneumonia. Experimental data20 suggest that influenza virus infection increases gene expression of antioxidants in the lungs. Influenza virus may have a direct effect on lung antioxidants gene expression and does not require the presence of innate immune response.19,20,43 According to our findings, in VCAP patients, the antioxidant status could be related with an increased activity of the redox glutathione system to a greater extent than SOD and CAT enzymes. An experimental study in mice20 observed that despite an increased expression of SOD mRNA, there was no increase in SOD activity in the lung homogenates from virus-infected animals. This antioxidant status could lead to a minor damage of biologic membrane and explain why VCAP patients had less organ failure when compared to those with BCAP.
The present study has several potential limitations that should be addressed. First, the number of patients included is limited and therefore, our results should be interpreted cautiously and further confirmed in a larger size of the population. In order to minimize a bias based on the number of patients included, patients with BCAP and VCAP were compared to a control group that comprised healthy volunteers and other non-infectious group of patients. In addition, age might be a confusion factor and to avoid a bias, control group was age-matched into two groups and compared to BCAP and VCAP patients. Second, this is an observational, not interventional study in which only adults with severe disease were included. In addition, only patients with viral infection due to 2009 A/H1N1 strain were studied, therefore our results might not be generalized to other populations with others virus strain and mild forms. Third, we did not measure other oxidant/antioxidant substances. However, oxidative stress can be investigated either by detection of oxygen free radicals and other similar substances (ROS), or by detection of antioxidants and damage products of essential biomolecules (e.g. lipid peroxidation products) as a consequence of ROS activity. Our research was performed according to the second investigation line.
In conclusion, our preliminary data might suggest the occurrence of pro-oxidant/anti-oxidant imbalance in CAP patients with respect to healthy volunteers. In contrast, a higher antioxidant activity in patients with 2009 A/H1N1 viral pneumonia was observed. Changes in antioxidant activity might be associated with a lower organ dysfunction according to the SOFA score. A better knowledge of the molecular mechanisms that sequentially regulate this battery of genes in relation to GSH levels in lung cells may open new therapeutic avenues in the modulation of inflammatory responses in lung diseases.
Financial disclosureDr Rodriguez, a time is partially protected by intensification line from the Instituto de Salud Carlos III (ISC III) – Programa I3SNS – INT11/239.
Supported in part by FIS PI10/01538.
The fund providers had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interestThe authors declare no conflicts of interest.
Partially presented at the 23rd Annual Congress of the European Society of Intensive Care Medicine. 2010, Barcelona, Spain.