To evaluate the feasibility and reproducibility of the scalenus anterior thickening fraction and its diagnostic accuracy to predict weaning outcomes.
DesignProspective observational study conducted as 2 sub-studies: sub-study A, which included healthy volunteers, and sub-study B, which included critically ill patients undergoing mechanical ventilation weaning.
SettingSingle-center study conducted at a tertiary center over 6 months.
ParticipantsTwenty-one healthy volunteers were included in sub-study A, whereas sub-study B included 66 critically ill patients undergoing weaning from mechanical ventilation.
InterventionsA high-frequency linear transducer was placed horizontally at the level of the cricoid cartilage with the neck rotated to the opposite side; at this point, the scalenus anterior muscle can be visualized clearly. The M mode was then switched on in the middle of the muscle with the sweep speed adjusted to a minimum to allow multiple breaths to be obtained on the same screen. Inspiratory and expiratory thickness was measured as an average of 3 breaths; subsequently, the thickening fraction was calculated as (inspiratory thickness – expiratory thickness)/expiratory thickness and expressed as a percentage.
Main variables of interestAccuracy of the scalenus anterior thickening fraction to predict failed weaning.
ResultsScalenus anterior thickening has good intra- and inter-observer reliability with interclass correlation coefficients of 0.79 and 0.8 for inspiratory and expiratory muscles, respectively. It also showed good accuracy in predicting failed spontaneous breathing trials with an area under the curve (95% confidence interval) of 0.92 (0.82−1) and 0.94 (0.84−0.98) for the right and left sides, respectively. Additionally, scalenus anterior thickening fraction could predict reintubation with an area under the curve of 1.00 (0.93−1.00) and 0.99 (0.91−1.00).
ConclusionScalenus anterior examination is a feasible tool with good interobserver reliability. The scalenus anterior thickening fraction could accurately predict weaning outcomes.
Evaluar la viabilidad y reproducibilidad de la fracción de engrosamiento del escaleno anterior y su precisión diagnóstica para predecir los resultados de la desconexión.
DiseñoEstudio observacional prospectivo, realizado en dos subestudios: el subestudio A, con voluntarios sanos, y el subestudio B, con pacientes críticos en desconexión de la ventilación mecánica.
ÁmbitoEstudio unicéntrico realizado en un centro terciario durante 6 meses.
Pacientes o participantesEl subestudio A incluyó a 21 voluntarios sanos, mientras que el subestudio B incluyó a 66 pacientes críticos en desconexión de la ventilación mecánica.
IntervencionesSe colocó un transductor lineal de alta frecuencia horizontalmente a la altura del cartílago cricoides con el cuello rotado hacia el lado opuesto; en este punto, se puede visualizar claramente el músculo escaleno anterior. A continuación, se activó el modo M en el centro del músculo con la velocidad de barrido ajustada al mínimo para permitir la obtención de múltiples respiraciones en la misma pantalla. El espesor inspiratorio y espiratorio se midió como un promedio de 3 respiraciones; posteriormente, se calculó la fracción de engrosamiento como (espesor inspiratorio - espesor espiratorio)/espesor espiratorio y se expresó como porcentaje.
Variables de interés principalesPrecisión de la fracción de engrosamiento del escaleno anterior para predecir el destete fallido.
ResultadosEl engrosamiento del escaleno anterior presenta una buena fiabilidad intra e interobservador, con coeficientes de correlación interclase de 0,79 y 0,8 para los músculos inspiratorio y espiratorio, respectivamente. También mostró una buena precisión en la predicción de intentos fallidos de respiración espontánea, con un área bajo la curva (IC del 95%) de 0,92 (0,82–1) y 0,94 (0,84–0,98) para el lado derecho e izquierdo, respectivamente. Además, la fracción de engrosamiento del escaleno anterior pudo predecir la reintubación, con un área bajo la curva de 1,00 (0,93–1,00) y 0,99 (0,91–1,00).
ConclusiónLa exploración del escaleno anterior es una herramienta viable con buena fiabilidad interobservador. La fracción de engrosamiento del escaleno anterior podría predecir con precisión los resultados del destete.
The decision to ventilate and eventually to extubate is an everyday practice in intensive care units. Despite its usefulness, prolonged use of mechanical ventilation (MV) results in several hazards. Conversely, premature separation of MV leads to reintubations with serious cardiorespiratory consequences and increased mortality.1 Failed weaning is a complicated, multifaceted event attributed to either direct respiratory or extraneous causes.2 Consequently, the timing for separation from mechanical ventilation must be thoroughly assessed; although various screening techniques have been proposed to predict weaning outcomes, including rapid shallow breathing index (RSBI), perfusion index, and dyspnea intensity.3–5 None has proven to be optimal thus far. Notably, ultrasound is a safe, noninvasive, and readily available bedside tool that provides immediate and dynamic information, making it particularly valuable in the critical care setting for assessing readiness for extubation. In this context, respiratory ultrasound evaluation emerged as an accurate, bedside, non-invasive, and easily repeatable tool, including assessment of lung parenchyma, diaphragm, and extra-diaphragmatic accessory muscles. Moreover, respiratory ultrasound guides the diagnosis and management of acute respiratory failure, improving clinical decision-making and patient outcomes.6 Nonetheless, there are some challenges, including inappropriateness for patients with elevated abdominal pressure, difficulty conducting left-side examinations, and a lack of predictive capability in specific populations.7–12 Furthermore, diaphragmatic assessments may be influenced by the administration of sedative medications and the existence of pain.13–15 There is a well-known inverse relationship between the diaphragm and accessory respiratory muscles; hence, in the case of diaphragmatic dysfunction, accessory respiratory muscles increase their activity to keep adequate tidal volume.7,8,16–18 The diagnostic accuracy of accessory respiratory muscles, such as the parasternal intercostal and abdominal muscles, in predicting weaning outcomes is becoming increasingly evident.16,19,20 The anterior scalene muscle is a superficial accessory inspiratory muscle that originates from the transverse processes of cervical vertebrae C3 to C6 and is inserted into the first rib.21 The scalene muscle electromyography, as suggested by recent evidence, has a positive correlation between electrical activity and dyspnea intensity.22 We hypothesized that the activity of the scalenus anterior muscle increases in patients with increased workload or diaphragmatic weakness; therefore, it can be used as a marker for weaning outcomes. To the best of our knowledge, no previous studies have investigated the role of scalenus anterior thickening as a predictor of weaning outcomes. Therefore, we aimed to evaluate the feasibility and reproducibility of scalenus anterior thickening as well as its ability to predict weaning outcomes.
Patients and methodsThis prospective observational study was carried out at a university hospital after approval from the institutional research ethics committee (MD-155-2024) and concordance with Helsinki's declaration. This study was conducted over six months from July 2024 to December 2024 and reported by the Strengthening Reporting of Observational Studies in Epidemiology (STROBE) guidelines. The patient or their next of kin signed an informed consent form before the commencement of the study.
This study was divided into two parts: sub-study A, which evaluated the feasibility and reproducibility of the right scalenus anterior thickening in healthy subjects, and sub-study B, which included sixty-six critically ill patients undergoing spontaneous breathing trials (SBT).
Sub-study A: Two experts in respiratory muscle ultrasonography conducted an exploratory study that included 21 healthy volunteers to determine the feasibility and reproducibility of scalene muscle evaluation. Each examined the right scalenus anterior thickness during inspiration and expiration, and the scalene thickening fraction was determined. All patients were given a thirty-minute rest before evaluation. Observer 1(MH) conducted two sets of examinations separated by a 10-minute delay; after 20 min, observer 2 (LM) performed comparable examinations. Both observers were blind to the measurements of the other. Intra and interobserver reliability were checked for right-side examinations.
Sub-study B: Included sixty-six adult critically ill patients who were mechanically ventilated for 24 h or more and were eligible for SBT. Patients fulfilling readiness for weaning criteria (Supplementary file) were subjected to SBT for 30 min using zero-pressure support and zero positive end-expiratory pressure, since our main objective was to evaluate the patient's intrinsic respiratory effort without the influence of ventilatory assistance as a confounding factor. Patients with neuromuscular disorders, tracheostomy, and limited ultrasound views (poor acoustic window, subcutaneous emphysema, surgical dressing, or central venous catheter at the site of examination) were not eligible. Failed weaning was defined as failed SBT or reintubation within 48 h. Criteria for SBT failure included increased heart rate > 140 beats/minute or respiratory rate > 35 breaths/min, significant blood pressure fluctuations, specifically systolic blood pressure (SBP) > 180 mmHg, SBP < 90 mmHg, and /or a deviation exceeding 20% from baseline values and signs of increased accessory muscle activity and sweating. As per our institutional post-extubation protocol, prophylactic application of non-invasive ventilation (NIV) was indicated for patients identified as high-risk, specifically those with chronic obstructive pulmonary disease, an APACHE II score greater than 12, a duration of mechanical ventilation exceeding seven days, and age over 65 years. Importantly, NIV was employed exclusively as a prophylactic measure and not as a rescue intervention in cases of post-extubation respiratory failure. This approach was intended to prevent confounding extubation failure rates.
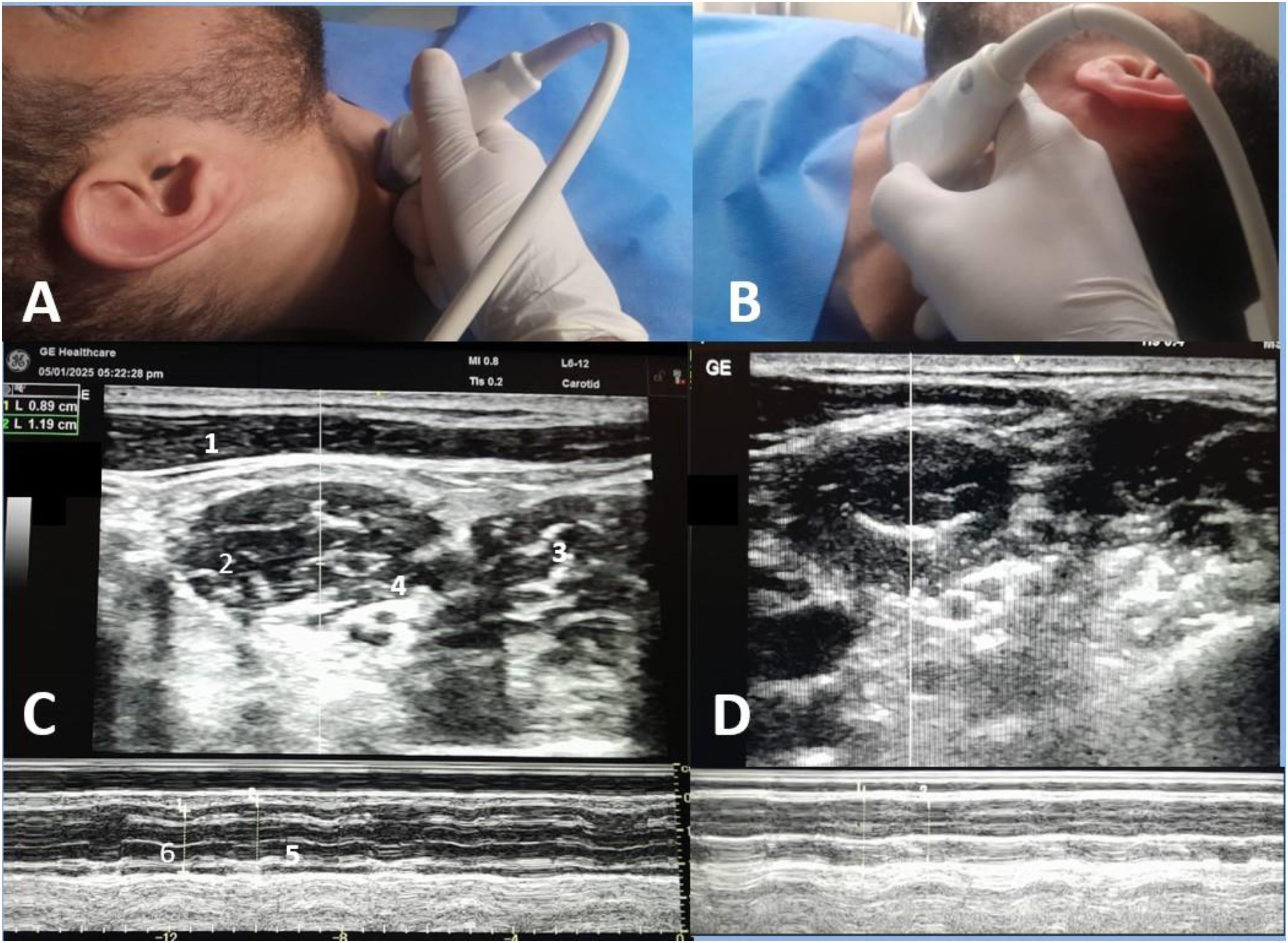
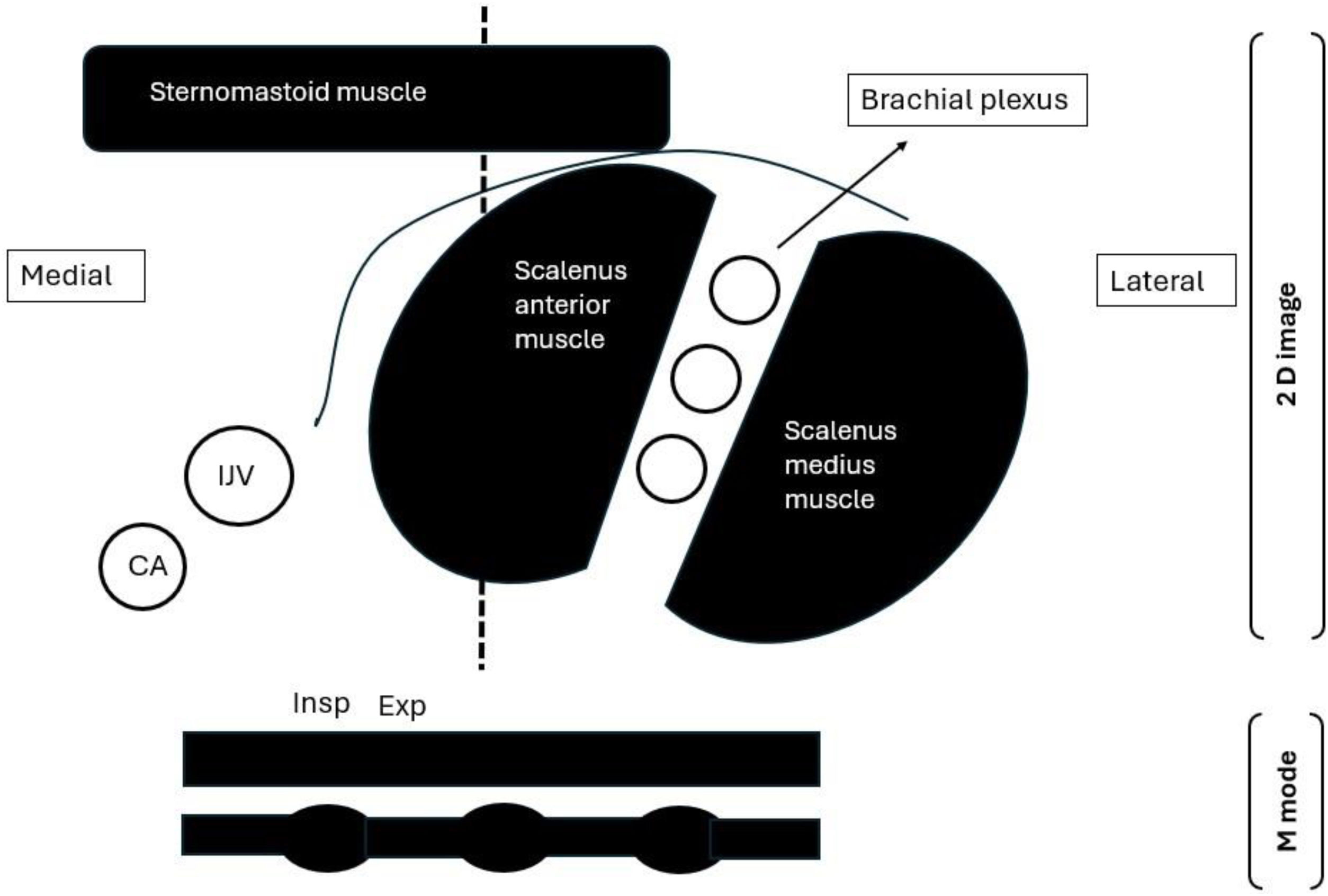
An experienced operator (AE), who completed more than 50 similar examinations before the commencement of the study, performed the diaphragmatic and scalene muscle ultrasound. The operator was blinded to the clinical data and not involved in the weaning process. Examinations were conducted using the Versana Essential device (GE Medical Systems Co., Ltd., China) at mid-trial (15 min after initiation of SBT) while the patient was semi-sitting. For scalene muscle evaluation, a high-frequency linear transducer (L6-12-RS, 4–16 MHz) was placed horizontally at the level of the cricoid cartilage with the neck rotated to the opposite side; at this point scalenus anterior muscle can be visualized clearly below the lateral border of sternocleidomastoid muscle, brachial plexus between the anterior and middle scalene muscles, and finally internal jugular vein below scalenus anterior muscle. The M mode was then switched on in the middle of the muscle with the sweep speed adjusted to a minimum to allow multiple breaths to be obtained on the same screen (Figs. 1, 2). Inspiratory and expiratory thickness was measured as an average of 3 breaths; subsequently, the thickening fraction was calculated as (inspiratory thickness – expiratory thickness)/expiratory thickness and expressed as a percentage. The diaphragmatic examination was in concordance with a recent consensus statement23 and current literature9,10,17 using a curved probe (4C-RS, 2–5 MHz) placed at the 10th or 11th intercostal at the right anterior axillary line and the left midaxillary line for each hemidiaphragm assessment, patient was then asked to take maximum breath, subsequently M mode was switched on to measure diaphragmatic excursion as an average of 3 successive breaths. The main outcome variable in this study was the ability of the scalenus anterior thickening fraction (SA-TF) to predict failed weaning (failed SBT and reintubation); other outcomes were the ability of RSBI to predict weaning outcomes, and finally, the correlation between diaphragmatic excursion and scalene thickening fraction.
Scalenus anterior thickening.
A-probe position for right side examination, B-probe position for left side examination.
C-ultrasound view of right scalenus anterior, D-ultrasound view of left scalenus anterior.
1-sternomastoid muscle, 2-anterior scalene muscle, 3-middle scalene muscle.
4-brachial plexus, 5-inspiratory thickness, 6-expiratory thickness.
Sub-study A: Being exploratory, previous similar studies16,19 were used to estimate the sample size; we therefore included 21 healthy subjects.
Sub-study B: Power analysis was performed using the Medcalc program (version 18) on the level of sensitivity of scalenus anterior thickening fraction to detect successful weaning from mechanical ventilation in an adult critically ill patient, using the ROC curve, because it was the primary outcome variable in this study. Based on the assumption that AUC is 0.7 and the null hypothesis is 0.5. A previous study that was carried out in the same unit showed that 56.6% of patients were successfully weaned from MV,24 and for a power of 0.8 and an alpha error of 0.05, a minimum sample size of 64 patients was calculated with at least 28 failed weaning cases.
For sub-study A, data were presented as mean (standard deviation) or median (quartiles) as appropriate and compared regarding gender. Intra and inter-observer reliability was checked for right scalene examinations using a one-way random-effects model. Consequently, inter-class correlation (ICC) coefficients were calculated using Medcalc software.
For sub-study B, patients were classified according to weaning outcomes into groups: successful weaning, failed SBT, and reintubation. Data were checked for normality using the Shapiro-Wilk test. Normally distributed data were reported as mean and standard deviation, while skewed data were reported as median and quartiles. An unpaired sample t-test and the Mann–Whitney test were used to compare both normally distributed and skewed data, respectively. Counts and frequencies were used to report categorical variables with a Chi-square for comparison. Receiver operating characteristic curves (ROC) were constructed, and the area under the curve (AUC) was calculated for SA TF, DE, and RSBI. The best cutoff values were calculated using the Youden index to predict failed SBT and reintubation. Correlation between diaphragmatic excursion and thickening fraction using Spearman correlation. Univariate logistic regression analysis was used to evaluate the association between individual clinical and physiological variables and weaning failure, defined as either failed SBT or the need for reintubation. The mean of right and left values was used to calculate the mean diaphragmatic excursion (M-DE). Similarly, the scalene thickening fraction (SA-TF) was measured bilaterally, and the average value was used to define the mean scalene thickening fraction (M-SA TF). Statistical analyses were performed using MedCalc Statistical Software version 18.2.1 (MedCalc Software Ltd., Ostend, Belgium) and IBM SPSS Statistics version 25.0 (IBM Corp., Armonk, NY, USA).
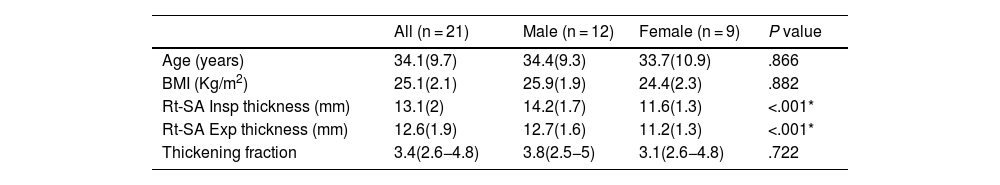
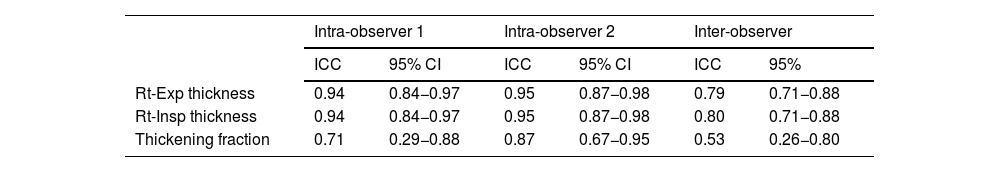
ResultsSub-study A: An Exploratory study included 21 healthy subjects, with 12 being males. The mean age for included subjects was 34.1 (9.7) years, and BMI was 25.1 (2.1). The inspiratory thickness of the right anterior scalene muscle was significantly greater in males (14.2 ± 1.7 mm) compared to females (11.6 ± 1.3 mm; P < .05). However, the absolute difference was relatively small, and given that our primary focus was on the thickening fraction rather than absolute thickness, this sex-based variation was not considered central to outcome prediction (Table 1). Intra-class correlation coefficient 0.94, 0.95 for observers 1 and 2, respectively. With interclass correlation coefficients of 0.79 and 0.8 for inspiratory and expiratory muscles, respectively (Table 2).
Baseline characteristics of healthy volunteers, data presented as mean (SD) and median (quartiles).
| All (n = 21) | Male (n = 12) | Female (n = 9) | P value | |
|---|---|---|---|---|
| Age (years) | 34.1(9.7) | 34.4(9.3) | 33.7(10.9) | .866 |
| BMI (Kg/m2) | 25.1(2.1) | 25.9(1.9) | 24.4(2.3) | .882 |
| Rt-SA Insp thickness (mm) | 13.1(2) | 14.2(1.7) | 11.6(1.3) | <.001* |
| Rt-SA Exp thickness (mm) | 12.6(1.9) | 12.7(1.6) | 11.2(1.3) | <.001* |
| Thickening fraction | 3.4(2.6−4.8) | 3.8(2.5−5) | 3.1(2.6−4.8) | .722 |
BMI: body mass index, Exp: expiratory, Insp: inspiratory, Rt SA: Right scalenus anterior.
Intra-observer and Inter-observer reliability.
| Intra-observer 1 | Intra-observer 2 | Inter-observer | ||||
|---|---|---|---|---|---|---|
| ICC | 95% CI | ICC | 95% CI | ICC | 95% | |
| Rt-Exp thickness | 0.94 | 0.84−0.97 | 0.95 | 0.87−0.98 | 0.79 | 0.71−0.88 |
| Rt-Insp thickness | 0.94 | 0.84−0.97 | 0.95 | 0.87−0.98 | 0.80 | 0.71−0.88 |
| Thickening fraction | 0.71 | 0.29−0.88 | 0.87 | 0.67−0.95 | 0.53 | 0.26−0.80 |
CI: confidence interval, ICC: inter-class correlation coefficient, Insp: inspiratory, Exp: expiratory, Rt: right.
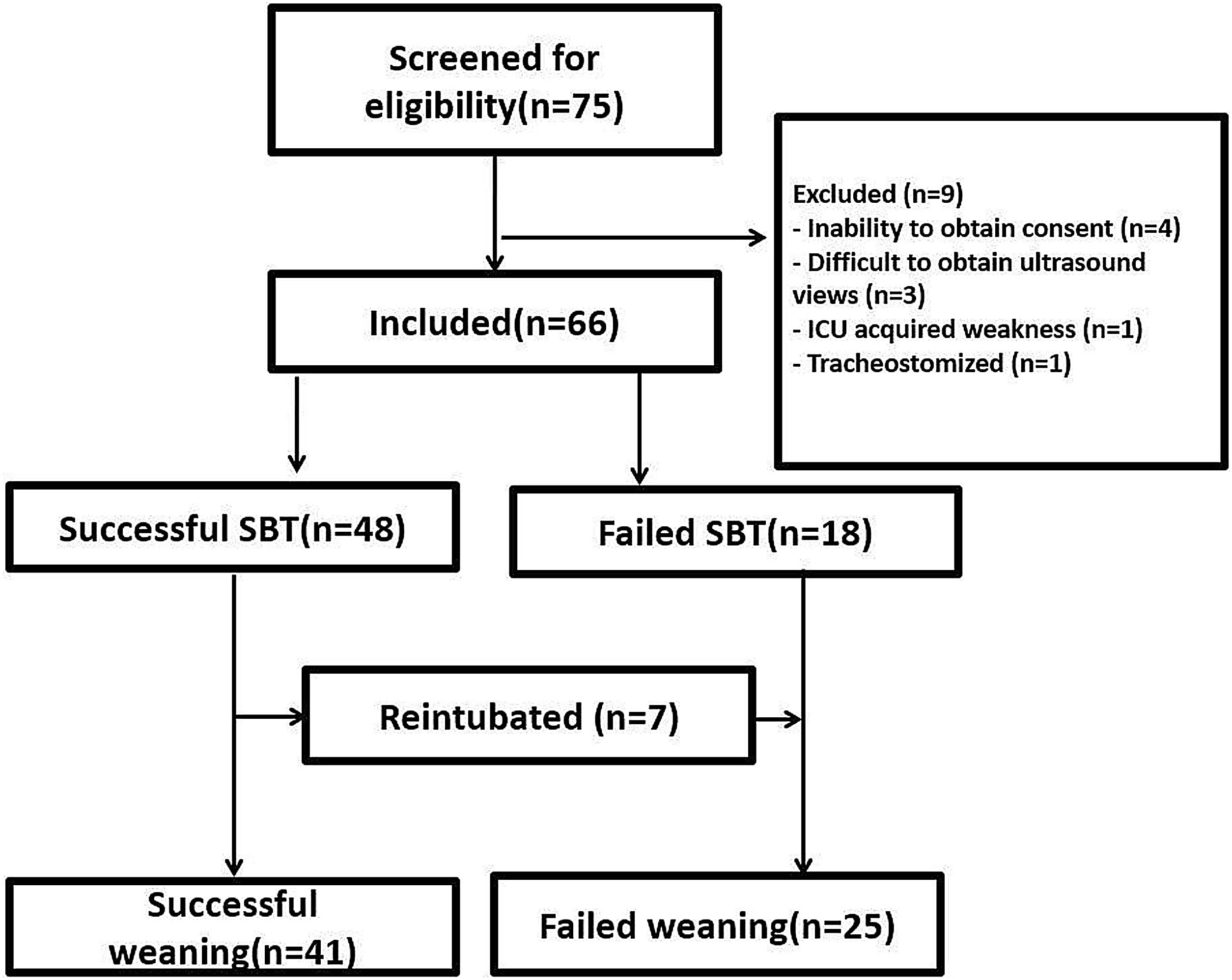
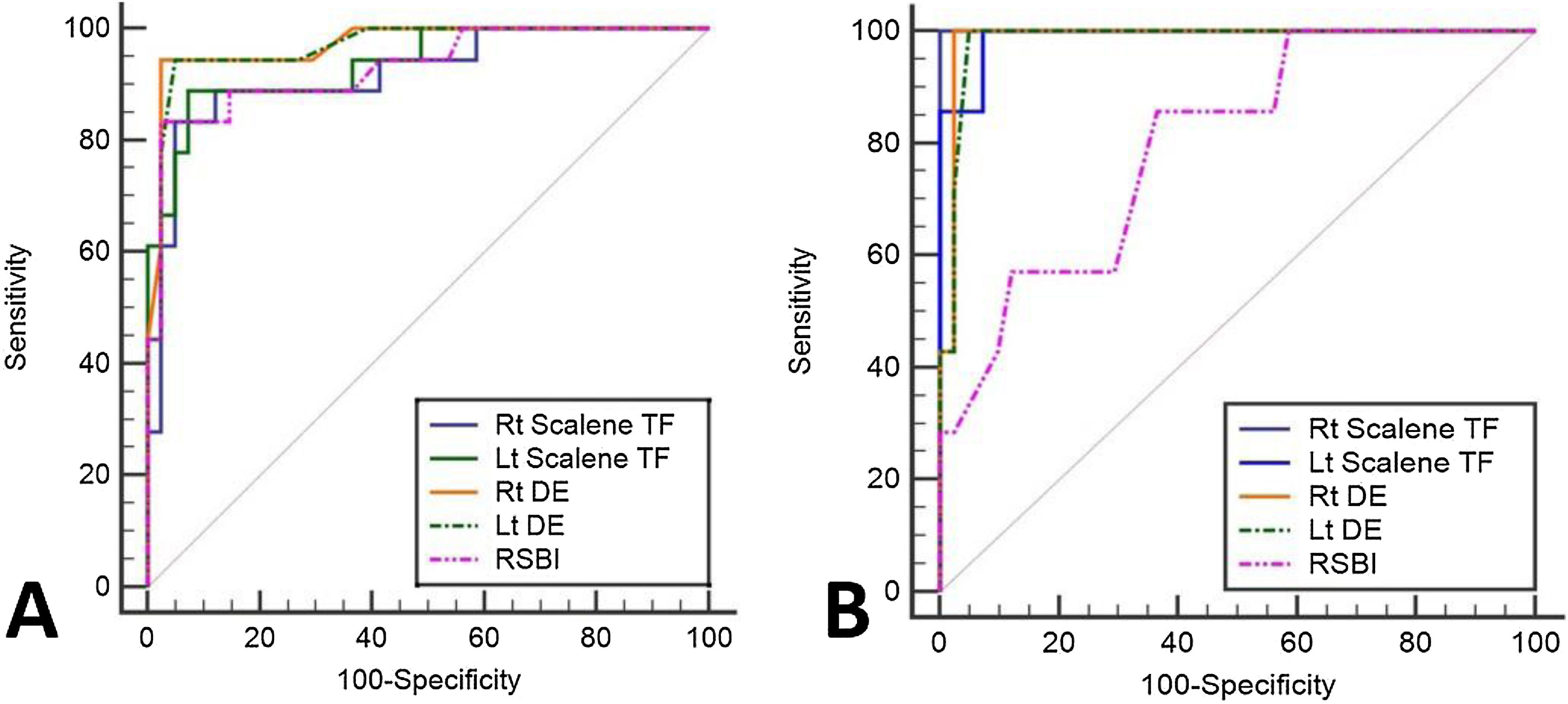
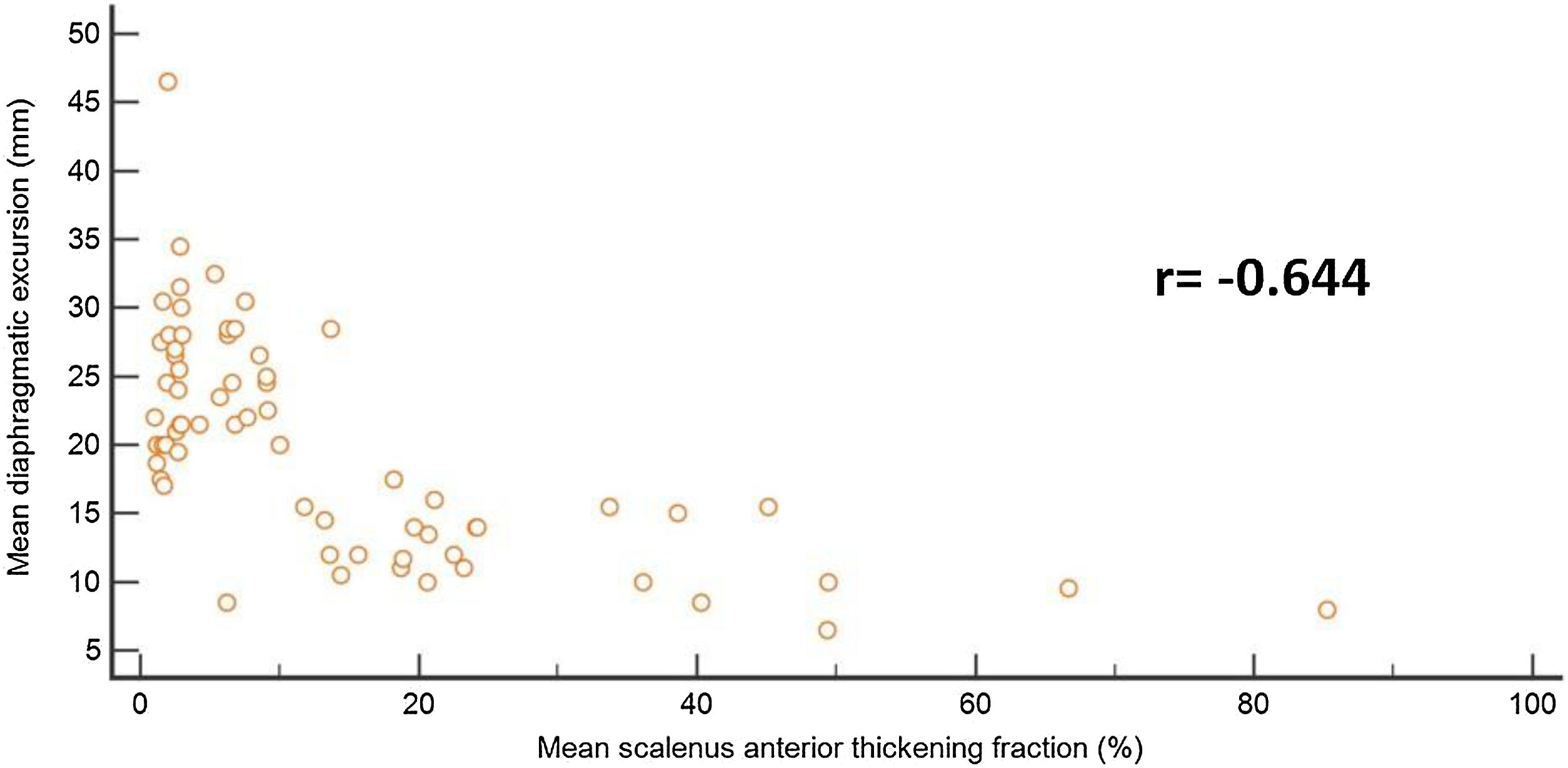
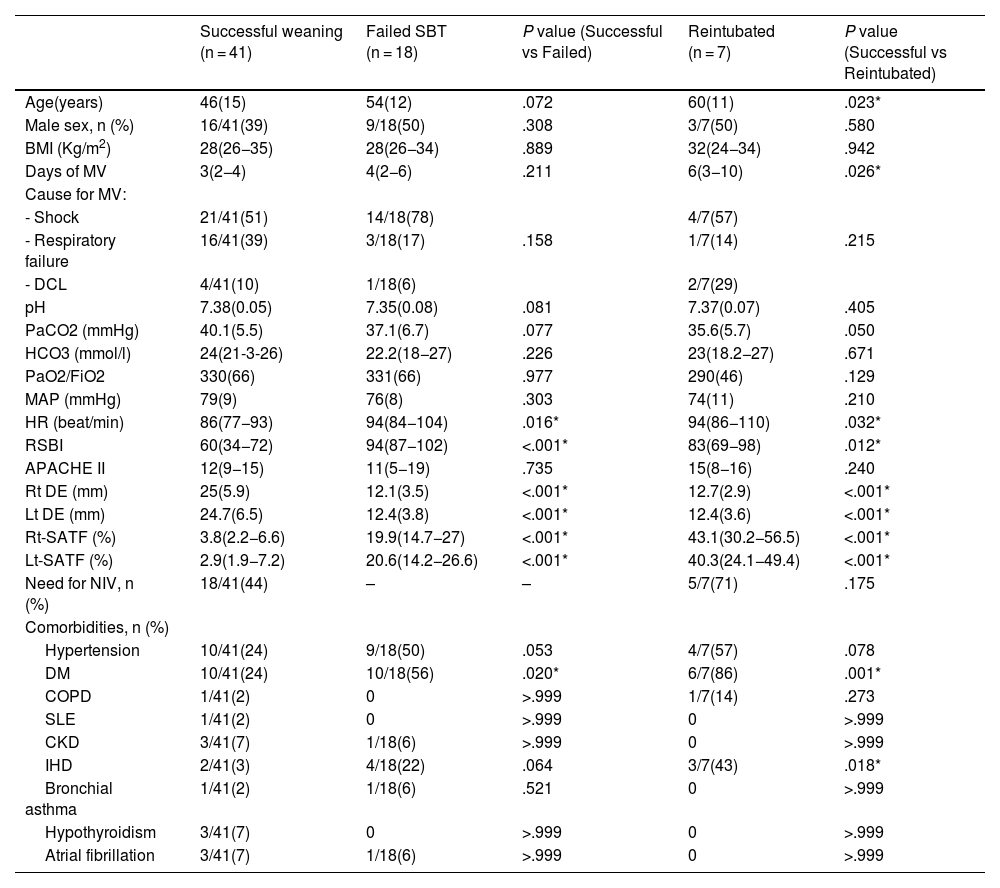
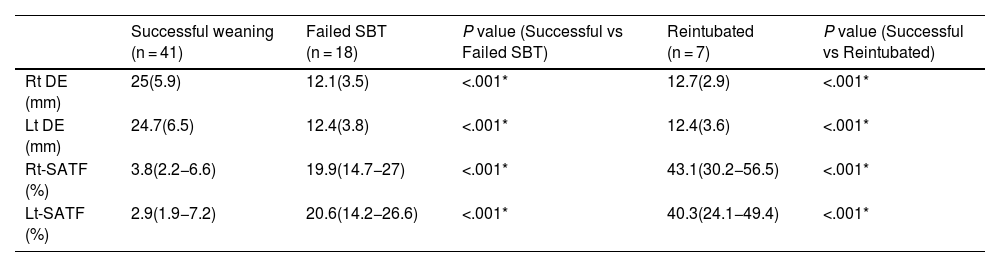
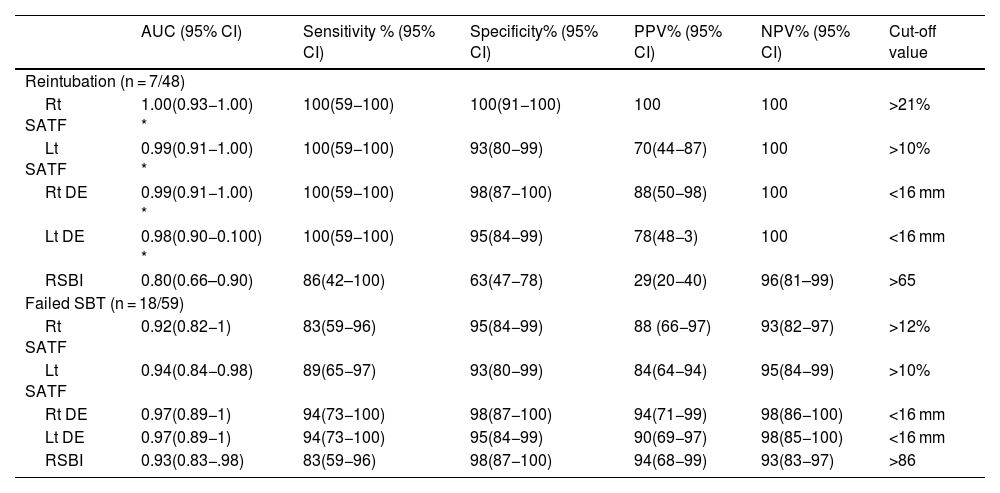
Sub-study B: Seventy-five critically ill patients were screened for eligibility; 7 patients were excluded, and 66 patients were available for final analysis; 41/66 (43%) showed successful weaning, 18/66 failed SBT, and 7/66 were reintubated (Fig. 3). Mean age and days of mechanical ventilation were higher in patients with reintubation. (Table 3) Patients who successfully weaned showed higher diaphragmatic excursion and lower SA-TF (Table 4). Patients’ comorbidities were summarized in Table 4. It is noteworthy that reintubated patients showed a significantly lower expiratory muscle thickness (Supplementary Table). Receiver operating characteristics (ROC) curve analysis for SA-TF revealed good accuracy for predicting SBT failure with an AUC (95% CI) of 0.92 (0.82−1) and 0.94 (0.84−0.98) for the right and left sides, respectively. Added to that, SA-TF could predict reintubation with an AUC of 1.00 and 0.99 for the right and left sides, respectively (Fig. 4) (Table 5). SA-TF < 10% can rule out weaning failure with 100% negative predictive value. Rt SA TF's reported cut-off values for predicting failed SBT and reintubations were 21% and 12%, respectively. While that of Lt SA TF was 10% (Table 5). Interestingly, there is a strong negative relationship between mean diaphragmatic excursion and mean scalene thickening (Fig. 5).
Demographic and comorbidities, data presented as mean (standard deviation), median (quartiles), and count (frequency).
| Successful weaning (n = 41) | Failed SBT (n = 18) | P value (Successful vs Failed) | Reintubated (n = 7) | P value (Successful vs Reintubated) | |
|---|---|---|---|---|---|
| Age(years) | 46(15) | 54(12) | .072 | 60(11) | .023* |
| Male sex, n (%) | 16/41(39) | 9/18(50) | .308 | 3/7(50) | .580 |
| BMI (Kg/m2) | 28(26−35) | 28(26−34) | .889 | 32(24−34) | .942 |
| Days of MV | 3(2−4) | 4(2−6) | .211 | 6(3−10) | .026* |
| Cause for MV: | |||||
| - Shock | 21/41(51) | 14/18(78) | 4/7(57) | ||
| - Respiratory failure | 16/41(39) | 3/18(17) | .158 | 1/7(14) | .215 |
| - DCL | 4/41(10) | 1/18(6) | 2/7(29) | ||
| pH | 7.38(0.05) | 7.35(0.08) | .081 | 7.37(0.07) | .405 |
| PaCO2 (mmHg) | 40.1(5.5) | 37.1(6.7) | .077 | 35.6(5.7) | .050 |
| HCO3 (mmol/l) | 24(21-3-26) | 22.2(18−27) | .226 | 23(18.2−27) | .671 |
| PaO2/FiO2 | 330(66) | 331(66) | .977 | 290(46) | .129 |
| MAP (mmHg) | 79(9) | 76(8) | .303 | 74(11) | .210 |
| HR (beat/min) | 86(77−93) | 94(84−104) | .016* | 94(86−110) | .032* |
| RSBI | 60(34−72) | 94(87−102) | <.001* | 83(69−98) | .012* |
| APACHE II | 12(9−15) | 11(5−19) | .735 | 15(8−16) | .240 |
| Rt DE (mm) | 25(5.9) | 12.1(3.5) | <.001* | 12.7(2.9) | <.001* |
| Lt DE (mm) | 24.7(6.5) | 12.4(3.8) | <.001* | 12.4(3.6) | <.001* |
| Rt-SATF (%) | 3.8(2.2−6.6) | 19.9(14.7−27) | <.001* | 43.1(30.2−56.5) | <.001* |
| Lt-SATF (%) | 2.9(1.9−7.2) | 20.6(14.2−26.6) | <.001* | 40.3(24.1−49.4) | <.001* |
| Need for NIV, n (%) | 18/41(44) | – | – | 5/7(71) | .175 |
| Comorbidities, n (%) | |||||
| Hypertension | 10/41(24) | 9/18(50) | .053 | 4/7(57) | .078 |
| DM | 10/41(24) | 10/18(56) | .020* | 6/7(86) | .001* |
| COPD | 1/41(2) | 0 | >.999 | 1/7(14) | .273 |
| SLE | 1/41(2) | 0 | >.999 | 0 | >.999 |
| CKD | 3/41(7) | 1/18(6) | >.999 | 0 | >.999 |
| IHD | 2/41(3) | 4/18(22) | .064 | 3/7(43) | .018* |
| Bronchial asthma | 1/41(2) | 1/18(6) | .521 | 0 | >.999 |
| Hypothyroidism | 3/41(7) | 0 | >.999 | 0 | >.999 |
| Atrial fibrillation | 3/41(7) | 1/18(6) | >.999 | 0 | >.999 |
APACHE II: acute physiology and chronic health evaluation, BMI: body mass index, CKD: chronic kidney disease, COPD: chronic obstructive pulmonary disease, DM: diabetes mellitus, DCL: disturbed conscious level, HR: heart rate, IHD: ischemic heart disease, MV: mechanical ventilation, NIV: non-invasive ventilation, RSBI: rapid shallow breathing index, SBT: spontaneous breathing trial, SLE systemic lupus erythematosus.
Ultrasound data for diaphragmatic excursion and scalenus thickening fraction, data presented as mean (SD) and median (quartiles).
| Successful weaning (n = 41) | Failed SBT (n = 18) | P value (Successful vs Failed SBT) | Reintubated (n = 7) | P value (Successful vs Reintubated) | |
|---|---|---|---|---|---|
| Rt DE (mm) | 25(5.9) | 12.1(3.5) | <.001* | 12.7(2.9) | <.001* |
| Lt DE (mm) | 24.7(6.5) | 12.4(3.8) | <.001* | 12.4(3.6) | <.001* |
| Rt-SATF (%) | 3.8(2.2−6.6) | 19.9(14.7−27) | <.001* | 43.1(30.2−56.5) | <.001* |
| Lt-SATF (%) | 2.9(1.9−7.2) | 20.6(14.2−26.6) | <.001* | 40.3(24.1−49.4) | <.001* |
Lt DE: left diaphragmatic excursion, Lt-SATF: left scalenus anterior thickening fraction, Rt DE: right diaphragmatic excursion, Rt SATF: right scalenus anterior thickening fraction, SBT: spontaneous breathing trial.
ROC analysis for the ability to predict reintubation.
| AUC (95% CI) | Sensitivity % (95% CI) | Specificity% (95% CI) | PPV% (95% CI) | NPV% (95% CI) | Cut-off value | |
|---|---|---|---|---|---|---|
| Reintubation (n = 7/48) | ||||||
| Rt SATF | 1.00(0.93−1.00) * | 100(59−100) | 100(91−100) | 100 | 100 | >21% |
| Lt SATF | 0.99(0.91−1.00) * | 100(59−100) | 93(80−99) | 70(44−87) | 100 | >10% |
| Rt DE | 0.99(0.91−1.00) * | 100(59−100) | 98(87−100) | 88(50−98) | 100 | <16 mm |
| Lt DE | 0.98(0.90−0.100) * | 100(59−100) | 95(84−99) | 78(48−3) | 100 | <16 mm |
| RSBI | 0.80(0.66–0.90) | 86(42–100) | 63(47−78) | 29(20−40) | 96(81–99) | >65 |
| Failed SBT (n = 18/59) | ||||||
| Rt SATF | 0.92(0.82−1) | 83(59−96) | 95(84−99) | 88 (66−97) | 93(82−97) | >12% |
| Lt SATF | 0.94(0.84−0.98) | 89(65−97) | 93(80−99) | 84(64−94) | 95(84−99) | >10% |
| Rt DE | 0.97(0.89−1) | 94(73−100) | 98(87−100) | 94(71−99) | 98(86−100) | <16 mm |
| Lt DE | 0.97(0.89−1) | 94(73−100) | 95(84−99) | 90(69−97) | 98(85−100) | <16 mm |
| RSBI | 0.93(0.83−.98) | 83(59−96) | 98(87−100) | 94(68−99) | 93(83−97) | >86 |
AUC: area under the curve, CI: confidence interval, Lt DE: left diaphragmatic excursion, Lt SATF: left scalenus anterior thickening fraction, NPV: negative predictive value, PPV: positive predictive value, RSBI: rapid shallow breathing index, Rt DE: right diaphragmatic excursion, Rt SATF: right scalenus anterior thickening fraction, SBT: spontaneous breathing trial, SLE: systemic lupus erythematosus.
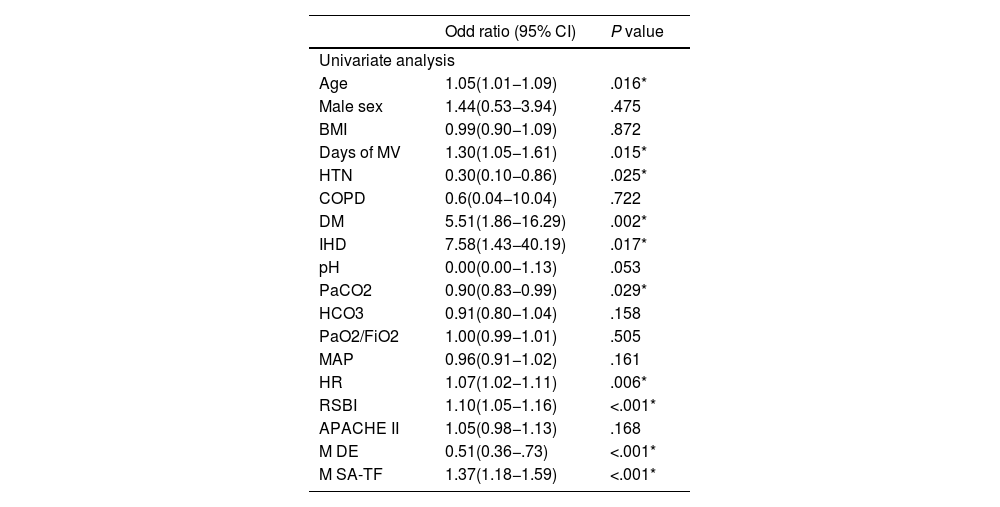
Notably, univariate analysis showed significant associations with several variables, including age (OR 1.05, 95% CI 1.01–1.09, P = .016), days of mechanical ventilation (OR 1.30, 95% CI 1.05–1.61, P = .015), diabetes mellitus (OR 5.51, 95% CI 1.86–16.29, P = .002), ischemic heart disease (OR 7.58, 95% CI 1.43–40.19, P = .017), and PaCO₂ (OR 0.90, 95% CI 0.83–0.99, P = .029). Increased heart rate (OR 1.07, 95% CI 1.02–1.11, P = .006) and elevated RSBI (OR 1.10, 95% CI 1.05–1.16, P < .001) were also significant. Importantly, a lower M-DE (OR 0.51, 95% CI 0.36–0.73, P < .001) and higher M-SA TF (OR 1.37, 95% CI 1.18–1.59, P < .001) were strongly associated with weaning failure (Table 6).
Univariate logistic regression analysis of variables associated with weaning failure.
| Odd ratio (95% CI) | P value | |
|---|---|---|
| Univariate analysis | ||
| Age | 1.05(1.01−1.09) | .016* |
| Male sex | 1.44(0.53−3.94) | .475 |
| BMI | 0.99(0.90−1.09) | .872 |
| Days of MV | 1.30(1.05−1.61) | .015* |
| HTN | 0.30(0.10−0.86) | .025* |
| COPD | 0.6(0.04−10.04) | .722 |
| DM | 5.51(1.86−16.29) | .002* |
| IHD | 7.58(1.43−40.19) | .017* |
| pH | 0.00(0.00−1.13) | .053 |
| PaCO2 | 0.90(0.83−0.99) | .029* |
| HCO3 | 0.91(0.80−1.04) | .158 |
| PaO2/FiO2 | 1.00(0.99−1.01) | .505 |
| MAP | 0.96(0.91−1.02) | .161 |
| HR | 1.07(1.02−1.11) | .006* |
| RSBI | 1.10(1.05−1.16) | <.001* |
| APACHE II | 1.05(0.98−1.13) | .168 |
| M DE | 0.51(0.36−.73) | <.001* |
| M SA-TF | 1.37(1.18−1.59) | <.001* |
APACHE II: Acute Physiology and Chronic Health Evaluation II score, BMI: body mass index, COPD: chronic obstructive pulmonary disease, DM: diabetes mellitus, HCO₃: bicarbonate, HR: heart rate, HTN: hypertension, IHD: ischemic heart disease, M DE: mean diaphragmatic excursion, M SA-TF: mean scalene thickening fraction, MAP: mean arterial pressure, MV: mechanical ventilation, PaCO₂: partial pressure of arterial carbon dioxide), PaO₂/FiO₂: arterial oxygen partial pressure to inspired oxygen fraction ratio, RSBI: rapid shallow breathing index, M-DE: mean diaphragmatic excursion, M-SA TF: mean scalenus anterior thickening fraction.
The current study demonstrated that the scalenus anterior thickening is a feasible and reproducible tool with good intra- and inter-observer reliability. Additionally, the scalene thickening fraction can accurately predict weaning outcomes. Notably, sex-based differences in scalene muscle thickness were observed; however, these are physiologically expected and unlikely to influence outcomes, as the thickening fraction, rather than absolute values, was used in our analysis.
This study is the first to evaluate anterior scalene muscle thickening as a predictor of weaning outcomes, offering novel insights into the potential role of superficial neck muscles in respiratory assessment. Given their accessibility and ease of ultrasound evaluation, this approach may be particularly advantageous in patients with abdominal or thoracic surgical interventions, or in those with increased abdominal distension, where traditional diaphragmatic imaging may be limited. Despite being a widely used tool to predict weaning outcomes, diaphragmatic ultrasound showed some challenges, including limited ultrasound views, not ideal for patients with abdominal surgery at or close to the site of assessment, difficulty in evaluating the left side, and being highly dependent on the level of pressure support.9,10,17,23,25,26 Therefore, accessory inspiratory and expiratory muscle evaluation has emerged as an alternative tool with comparable diagnostic accuracy.7,16,19 We report a strong negative relationship between diaphragmatic excursion and scalenus anterior thickening fraction; previous literature reported a similar negative relationship between diaphragmatic function and accessory respiratory muscles.16,19,20 This occurs because, in the case of diaphragmatic dysfunction, the accessory respiratory muscles increase their activity to compensate for both the increased workload and the diaphragmatic dysfunction, thereby maintaining adequate tidal volume. In line with our results, a recent physiologic study reported increased scalene electric activity with increased respiratory workload.22 The performance of abdominal expiratory muscles may be restricted due to recent abdominal surgery or elevated intra-abdominal pressure; also, the assessment of parasternal intercostal muscles is sensitive to the level of pressure support.27
LimitationsThis study was conducted at a single center with a relatively small sample size. Second, we did not examine the lung parenchyma; therefore, future studies are required to evaluate the combined evaluation of scalenus muscle thickening and lung ultrasound scores. Third, anatomical factors, including central lines, neck edema, and excessive soft tissue, may limit visualization of the scalene muscle. Finally, although univariate analysis identified key predictors of weaning failure, multivariable regression was not feasible due to quasi-separation arising from a small sample size and low event rate. This statistical constraint limits the ability to adjust for confounders and accurately assess independent effects. Larger future studies are needed to support comprehensive modeling and validate these associations.
ConclusionThe anterior scalene muscle thickening fraction (SA-TF) appears to be a feasible and reproducible ultrasound parameter, with good intra- and inter-observer reliability. In critically ill patients ventilated for ≥24 h, SA-TF showed promising predictive value for weaning outcomes. Although an SA-TF < 10% was associated with a high negative predictive value for weaning failure, these findings should be interpreted cautiously pending external validation and adjustment for confounding variables in future studies.
CRediT authorship contribution statementM.H.: This author helped in study design, data acquisition, and manuscript review. L.M.: This author helped in study design, data acquisition, and manuscript review. A.S.: This author helped in the conception of the idea, study design, analysis of the data, and drafting the manuscript. A.E., W.H.: These authors helped in study design, data acquisition, and manuscript review. All authors approved the manuscript and agreed to be accountable for all aspects of the work.
Ethics declarationEthical approval for this study was provided by the institutional research board of Cairo University Hospitals (MD-155-2024).
Consent to participateInformed consent was obtained from each patient or their next of kin before participation.
Consent for publicationNot applicable.
Declaration of Generative AI and AI-assisted technologies in the writing processGrammarly and Quillbot software were used for English editing.
FundingThe authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Data availabilityData regarding this study is available from the corresponding author upon reasonable request.
The authors have no relevant financial or non-financial interests to disclose.
None.
This study was conducted at Cairo University Hospital, Cairo University, Egypt.