Edited by: Federico Gordo - Medicina Intensiva del Hospital Universitario del Henares (Coslada-Madrid)
Last update: December 2023
More infoEnterobacteriaceae are the most frequent pathogens in the Intensive Care Unit. Due to their safety and activity, β-Lactams (BL) and carbapenems represented the most common strategy adopted against these germs. The increasing exposure to these molecules led to the development of several types of antimicrobial resistance as the expression of extended-spectrum β-lactamases (ESBLs) and carbapenemases. Great molecular variability exists among these enzymes, with significant clinical impact.
To limit morbidity and mortality, old antibiotics were tested and represent viable alternatives for specific types of infections, or once the spectrum of susceptibility of each germ has been determined. Alongside, new molecules have been specifically designed but enzyme molecular variability prevents the existence of one single antibiotic which fits for all.
Therefore, a quicker identification of the molecular identity of each germ, together with the knowledge of the activity spectrum of each antibiotic is crucial to tailor the therapy and make it effective.
Las enterobacterias son patógenos cada vez más frecuentes en las unidades de cuidados intensivos. Los antibióticos beta lactámicos y carbapenémicos representan las estrategias más comunes contra estos gérmenes, debido a sus mecanismos de acción y seguridad clínica. La exposición cada vez mayor de los patógenos a dichas moléculas ha llevado al desarrollo de nuevas diferentes resistencias a los antibióticos, representadas por la expresión de las beta lactamasas de espectro extendido y de las carbapenemasas. Esas enzimas manifiestan mucha variabilidad molecular, que resulta en un sustancial impacto clínico.
Una opción disponible y válida para limitar la morbilidad y la mortalidad de estas infecciones es volver a utilizar los viejos antibióticos, una vez que se haya averiguado el espectro de sensibilidad de los gérmenes. Además, nuevos antibióticos han sido específicamente diseñados para solucionar el problema de las resistencias. Sin embargo, la variabilidad molecular de las enzimas hace que sea muy difícil encontrar una única molécula que funcione para todas.
Por lo tanto, una rápida identificación de la identidad molecular de los gérmenes, junto a la comprensión detallada del espectro de actividad de cada antibiótico, es de vital importancia para adaptar el tratamiento y hacerlo más efectivo.
The emergence and wide spread of multi-drug resistant (MDR) pathogens represent a clinical challenge for patients, physicians and health care systems. In this setting, β-Lactams (BL) are the oldest and most frequently prescribed antibiotics in daily clinical practice, which extended use has been identified as the predominant determinant of acquired bacterial resistance towards these drugs.1 Specifically, this condition was first described nearly 70 years ago in a strain of Escherichia coli, which was characterised by a specific hydrolytic enzyme that conferred resistance to β-Lactams, which was called β-Lactamases.2
In critical care practice, the EPIC III study3 reported that Gram-Negative bacteria and, specifically, the Enterobacteriaceae, were the most frequently isolated pathogens in critically ill patients admitted to the Intensive Care Unit (ICU) in 2017. Moreover, this study revealed that infections caused by Gram-Negative bacteria producing extended-spectrum β-lactamases (ESBLs) and carbapenemases were significant risk factors for hospital mortality. These findings were justified by the impact of MDR pathogens on the delivery of effective antimicrobial therapy, leading to delayed administration of appropriate antibiotics with consequent worse clinical outcomes.4 Furthermore, the use of carbapenems to treat infection caused by ESBLs-producing pathogens grew after the publication of the MERINO study,5 a randomised clinical trial that did not demonstrate the inferiority of piperacillin-tazobactam compared with meropenem.6 For these reasons, carbapenems sparing therapeutic option are strongly advocated and the World Health Organization listed the development of new effective drug on ESBL and carbapenemases-producing Enterobacteriaceae as a research priority in order to modulate the increased morbidity and mortality caused by such pathogens.7
In this paper, we reported an updated review of the literature on the mechanisms of resistance of ESBLs and carbapenemases-producing Enterobacteriaceae. Moreover, we provided an overview of therapeutic strategies for the management of severe infections caused by these pathogens with a brief presentation of “old” and “new” antibiotics used in this setting.
ESBL-producing EnterobacteriaceaeMechanism of antibiotic resistanceESBL represents a group of enzymes that hydrolyses and confers resistance to a variety of β-lactams, like third generation cephalosporines (i.e., cefotaxime, ceftriaxone and ceftazidime) and monobactams (i.e., aztreonam), with the exception of cephamycins (i.e., cefoxitin, cefotetan) and carbapenems (i.e., imipenem, meropenem, doripenem, ertapenem).8 This group accounts for several enzymes that may be classified according to the amino acid sequence homology into the “molecular” Ambler classes A to D.9 Although this classification is the most widely used in clinical practice, ESBLs may be graded according to the substrate and inhibitors profiles into the “functional” Bush-Jacoby-Medeiros groups 1, 2 and 3.10 From an epidemiological point of view, the majority of ESBLs belongs to the Ambler class A and are codified by the SHV, TEM and CTX-M genes. Over the past decades, Klebsiella Pneumoniae and E. Coli have been observed as the most common ESBLs-producing Enterobacteriaceae (ESBL-E) isolated worldwide from hospitals, among which CTX-M is prevalent.11 Moreover, CTX-M derived ESBL-E have been identified in E. coli strains from community sites, for which co-resistance for other classes of antibiotics like cotrimoxazole, tetracycline, gentamicin, and ciprofloxacin was the main reason for therapeutic failure and worse clinical outcome when cephalosporins and fluoroquinolones were used as empirical antibiotics. Moreover, recent evidence reported that some SHV and TEM derived β-lactamases are characterized by amino acid substitutions that confer resistance to the β-lactamase inhibitors clavulanate and sulbactam, while remaining susceptible to tazobactam and avibactam.12
On top of that, the in-vitro efficacy of some β-lactams and β-lactamase inhibitor (BLBLI) may be limited by the “inoculum effect”, which is caused by the proportional relationship between the minimum inhibitory concentration and bacterial load.13 Finally, ESBL-E may have additional genes or mutations in genes that mediate resistance to a broad range of antibiotics (cotrimoxazole, tetracyclines, aminoglycosides, and fluoroquinolones), thus challenging the prescription of appropriate antibiotic therapy.14
Old moleculesCarbapenems represent the first choice to treat infections caused by ESBL-E because they are not susceptible to enzymatic hydrolysis, are well distributed at high concentration into body tissues and are not hampered by the inoculum effect. However, their pivotal role in the management of severe infections caused by ESBL-E has been questioned by high cost and the potential risk of favouring the growth of yeast and bacteria resisitant to these antibiotics. Moreover, the superiority of carbapenems over other molecules remains mainly theoretical and supported by comparative studies with high risk of bias.8 Specifically, the INCREMENT study collected retrospective data on 1004 episodes of blood stream infections due to ESBL-producing Enterobacteriaceae from 7 tertiary hospitals in 11 different countries between 2004 and 2013.15 The authors found that BLBLI, aminoglycosides and fluoroquinolones were not inferior to carbapenems when pathogens showed in-vitro susceptibility to these drugs and such findings were supported by a recent systematic review and meta-analysis on 35 papers on this topic.16 Consequently, these antibiotics may represent an option for de-scalation of empiric antibiotic therapy as soon as the spectrum of pathogen susceptibility to antibiotics is detected and their early use may be favoured by the implementation of rapid diagnostic tools of genomic analyses.17 On the contrary, the MERINO randomised clinical trial compared the effect of piperacillin/tazobactam (4.5 g every 6 h) with meropenem (1 g every 8 h) for the treatment of bloodstream infections due to cephalosporin resistant Enterobacterales in 379 patients from 26 hospitals in 9 countries between 2014 and 2017.5 The authors found that piperacillin/tazobactam was not non-inferior compared with meropenem with regards to 30-day mortality (12.3% vs. 3.7%, respectively; risk difference 8.6%, p = 0.9 for noninferiority). However, the results of this trial were hampered by several drawbacks like the premature termination of the study and the wide post-hoc detected non-susceptibility of microbiological isolates to piperacillin-tazobactam. Specifically, in a MERINO subgroup analysis, Henderson et al.18 found a decreasing difference of absolute risk of 30-day mortality when piperacillin-tazobactam resistant strains were excluded. Nevertheless, a difference still exists and further trials are underway to elucidate this problem.19 As it stands, carbapenems use appears reasonable in patients with severe infections caused by ceftriaxone-resistant Enterobacteriaceae, although other therapeutic options should be driven by in-vitro spectrum of antibiotic susceptibility. Although meropenem is the most studied carbapenem, ertapenem is also indicated for EBSL-E pyelonephritis and complicated urinary tract infections, even though a randomized controlled trial demonstrated piperacillin-tazobactam non-inferiority.20 Other antibiotics may play a role in the management of ESBL-producing Enterobacteriaceae, although their effect has never been tested in randomised clinical trials. As a matter of fact, cefepime, a fourth generation cephalosporine, shows potent in-vitro activity against these pathogens, although it is hampered by the “inoculum effect” and clinical studies showed that it was associated with higher treatment failure compared with carbapenems or piperacillin-tazobactam.20,21
Apart from BLBLI, aminoglycosides and fluoroquinolones, other carbapenem-sparing options in the management of ESBL-E are represented by tigecycline, fosfomycin and trimethoprim-sulfamethoxazole. Tigecycline may play a role in intra-abdominal (IAI) and soft tissue infections (SSTI), although its low blood concentration limits its use for blood stream infection (BSI). On the contrary, high doses of fosfomycin (16−24 g daily) appeared useful to treat urinary tract infections (UTI), but a recent randomised clinical trial did not demonstrate noninferiority of this drug compared with meropenem, mainly due to treatment discontinuation caused by the development of adverse event.22 Finally, trimethoprim-sulfamethoxazole is recommended by IDSA guidelines for the management of uncomplicated UTIs or eventual cUTI caused by ESBL-E that are not resistant to this drug.23
New moleculesOver the past decade, new molecules as well as BL and carbapenems/BLI combinations have been implemented in clinical practice with the aim to treat severe infections caused by ESBL-E.
Among new molecules, cefiderocol is a cephalosporine that penetrates the pathogen via an iron transporter and is currently approved from the treatment of complicated UTI.24 Moreover, the two new tetracyclines, omadacycline and eravacycline, were approved for the treatment of community-acquired pneumonia, SSTI, and cIAI, although they have no activity against Pseudomonas Aeruginosa.25 On the contrary, plazomicin is a novel aminoglycoside that has a broad-spectrum in vitro activity against Enterobacteriaceae and Gram-positive bacteria and was demonstrated non-inferior to levofloxacin and meropenem to treat cUTI.26,27
New BLBLI combinations are represented by ceftazidime-avibactam and ceftolozane-tazobactam. Ceftazidime-avibactam is active against ESBLs (Ambler class A, C and D) Enterobacteriaceae and was demonstrated not inferior compared with carbapenems in the management of cIAI, cUTI, and nosocomial pneumonia.28–30
Ceftolozane-tazobactam is a fifth-generation cephalosporin which is active against ESBL-E. At the moment, it represents the most active antibiotic against Pseudomonas Aeruginosa and was approved for the treatment of nosocomial pneumonia, UTI and IAI in combination with metronidazole.31–33
Several subgroup or retrospective analyses confirm ceftolozane-tazobactam usefulness; clinical cure rate and microbiological response are equally high when compared to carbapenems.34 However, in-vitro susceptibility widely varies depending on the population studied and the germs involved,35 and frequent situations as septic shock and renal replacement therapy represent risk factors for non-response.36 Those drugs have a potential role in a carbapenem-sparing point-of-view, but the observations reported are controversial, especially when in-vitro susceptibility is not available. Hence, a direct comparison of BLBLI to carbapenem in a randomized control trial is needed. In this perspective the MERINO-3 trial is comparing ceftolozane-tazobactam to meropenem in ESBL-E bloodstream infections with expected conclusion in 2024.37
Finally, the new carbapenems/BLI combinations imipenem-relebactam and meropenem-vaborbactam may play a role in the management of severe infections caused by ESBLs-producing pathogens, although they represent the mainstay of therapy for carbapenemases-producing Enterobacteriaceae and will be described in detail over the next paragraphs.
Carbapenemases-producing EnterobacteriaceaeMechanism of antibiotic resistanceCarbapenem-resistant Enterobacteriaceae (CRE) are defined as bacteria resistant to at least one carbapenem antibiotic or producing a carbapenemase enzyme.23 Apart from carbapenemases, strategies for carbapenem-resistance include alterations to the target protein, an increase in efflux pumps or a decrease in membrane permeability.38 Carbapenemase-producing Enterobacteriaceae account approximately for 85% of all CRE, with wide geographical variation.39
Carbapenemases are protein which can break the molecular structure of antibiotics, avoiding their binding to the penicillin-binding protein. They are active against a variety of antibiotic classes, such as cephalosporins, penicillins, monobactams and carbapenems.40 According to their molecular structure, carbapenemases are found within class A, B and D of the Ambler classification system.9 Discovered in 1996 in a K. Pneumoniae isolate, KPC is the most frequent enzyme of Amber class A,41 has worldwide spread and is also expressed by other Enterobacteriaceae.42 Amber class B includes metallo-β-lactamases such as VIM, which is frequent in Southern Europe, IMP, accounting for 15% of CRE in Japan and Australia, and NDM which has the highest burden in Middle East and East Europe. OXA-48-like enzymes is a group of Ambler D class protein similar to OXA-48 enzyme, largely found in Acinetobacter. Variants in this group vary for geographical distribution and resistance spectrum, but they are found mostly in Northern Africa and Middle East.39,43
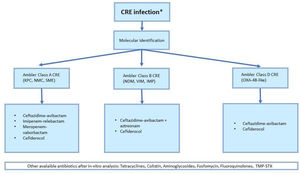
Clinical indication for antibiotic treatment of CRE infections depends on germ susceptibility and type of carbapenemase expressed. Therefore, molecular identification is crucial for an appropriate antibiotic regimen (Fig. 1).
Old moleculesSince the emergence of MDR, together with the development of new active antibiotics, research found new application for well-known and safe molecules. In fact, CRE are susceptible to old classes of drugs like monobactams, polymyxins, tetracyclines, aminoglycosides and quinolones.
Tetracycline role is interesting as susceptibility to these agents is independent of the presence or type of carbapenemase.44,45 Both carbapenemase-producing (e.g., KPC, NDM, OXA-48-like carbapenemases) and non-carbapenemase-producing Enterobacteriaceae may be susceptible to these molecules. Tigecycline has very low rate of in-vitro resistance (<1,3% of Enterobacteriales isolates) but its role in clinical practice is limited by poor benefit as monotherapy and in combination.46 Recent IDSA guidelines recommend tetracycline derivatives only as alternative options for IAI, SSTI, osteomyelitis and respiratory infections, when BL agents are inactive or not tolerated.23 The rationale behind this recommendation is that their rapid tissue distribution results in a poor concentration in urine or serum, making their use for urinary and bloodstream infections inappropriate.
The optimal dosing has now been shown to be 200 mg intravenously as a single dose followed by 100 mg intravenously every 12 h.47
Polymyxins, especially polymyxin E (colistin), have been widely used for CRE infections in the last twenty years, as a “revival” of old molecules in the era of multidrug resistant bacteria.48,49
However, several observational studies and RCT demonstrated their inferiority compared to other CRE-active agents, due to increased mortality and nephrotoxicity50–52 (Table 1). Moreover, in-vitro efficacy to colistin can be often limited by the above mentioned “inoculum effect”. Therefore, IDSA recommends against the use of intravenous polymyxins for the treatment of CRE infections, except for the uncomplicated CRE UTI, and only relating to polymyxin E (colistin) 23.
Outcome and side effects in clinical trials involving colistin.
| Authors | Type of study | Comparison | Primary outcome/Major finding | Side effects | Notes |
|---|---|---|---|---|---|
| Cisneros et al., 2021 | Randomized, controlled | Colistin vs. meropen (both plus levofloxacin) | 28-day mortality (23% vs. 25%) | Nephrotoxicity* (33% vs. 19%);CRRT* (9% vs. 2%) | Interrupted for excessive side-effectsHeterogeneous isolated germs (16% Pseudomonas Aeruginosa, 16% Acinetobacter spp, 14% Klebsiella spp) |
| Motsch et al., 2020 | Randomized, controlled | Imipenem-relebactam vs. imipenem plus colistin | Favorable overall response (71% vs. 70%) | Serious Adverse Events* (10% vs. 31%); Nephrotoxicity* (10% vs. 56%) | Isolated germs: 77% Pseudomonas Aeruginosa, 16% Klebsiella spp., 6% Other Enterobacteriaceae |
| van Duin et al., 2018 | Subgroup analysis (CRACKLE trial) | Ceftazidime-avibactam vs. colistin | 30-day mortality* (9% vs. 32%) | Nephrotoxicity (5% vs. 13%) | Most patients received additional anti-CRE agents as part of their treatment |
| Hakeam et al., 202 | Retrospective | Ceftazidime-avibactam vs. colistin | 14-day mortality (19% vs. 31%) | Nephrotoxicity (9% vs. 10%); CRRT (6% vs. 10%) | Most patients received additional anti-CRE agents as part of their treatment (72% vs. 100%) |
| Feng et al., 2021 | Retrospective | One effective antibiotic with or without aerosolized colistin | 14-day clinical failure rate* (35% vs. 57%) | Newly onset dialysis* (4% vs. 13%) | Different number of patient in two group; 80% of infections caused by Acinetobacter Baumanii |
| Tumbarello et al., 2013 | Retrospective | IV plus aerosolized colistin vs. IV colistin | Clinical cure rate* (69% vs. 55%) | New onset AKI (25% vs. 22%) | Days of mechanical ventilation* (8 vs. 12) |
CRRT, continuous renal replacement therapy; AKI, acute kidney injury.
Besides its nephrotoxicity, intravenous administration of colistin results in lack of lung tissue penetration and is therefore not recommended as a drug of choice for nosocomial pneumonias caused by CRE. On the other hand, animal studies showed a higher lung tissue concentration and bactericidal effect of nebulized colistin administration, making sense out of its aerosol delivery. However, there is not strong evidence regarding the effects of nebulized antibiotics, supported only by small observational studies with conflicting findings.53,54 Although the European Society of Clinical Microbiology and Infectious Disease guidelines55 recommend against adjunctive nebulized colistin in CRE-caused nosocomial pneumonia, the International Consensus Guidelines for the Optimal Use of the Polymyxins56 raises the concern that the benefits may overweight the risks, potentially laying the foundations for new recommendations.
In addition to colistin, fosfomycin, nitrofurantoin, aminoglycosides as gentamycin, amikacin and plazomicin and fluoroquinolones may be effective treatment options for uncomplicated UTIs caused by CRE.57,58
Fosfomycin should be administered as a single oral dose only as a treatment for uncomplicated CRE cystitis caused by E. Coli, because the fosA gene (that characterizes Klebsiella spp., Enterobacter spp. and Serratia Marcescens) can hydrolyse the molecule leading to clinical failure.23
Both nitrofurantoin and fosfomycin should be avoided for complicated UTI and pyelonephritis, due to their poor concentrations achieved in the renal parenchyma.
However, its in-vitro activity against CRE and other MDR bacteria leads to growing interest for intravenous use of this molecule.59
Monobactams as aztreonam are effective only against bacteria producing class B or D carbapenemases, with no activity against bacteria producing class A carbapenemases, including the highly prevalent KPC carbapenemases.
Aztreonam is indicated as an adjunctive drug together with new BLBLI combination in CRE infection in which metallo-β-lactamases are recognized, such as Enterobacterales producing NDM.
Although aztreonam is not hydrolyzed by NDMs, it should be avoided in monotherapy because ESBLs, AmpC β-lactamases and OXA-48-like carbapenemases are frequently co-produced by NDM-producing isolates.23
New moleculesTogether with the rediscovery of antibiotics which can retain anti-CRE activity, new molecules have been studied. Drugs which principally demonstrated to be appropriate for MDR organism are BLBLI combinations, but also other classes such as tetracyclines, cephalosporines and aminoglycosides have demonstrated to be non-inferior to carbapenems or penicillins for specific kind of infections.
BLI are molecules that can inhibit β-lactamases and carbapenemases restoring the activity of carbapenem or penicillin. Since resistance to clavulanate and sulbactam is recently increasing,12 novel molecules as avibactam, vaborbactam and relebactam have been developed.
One of the most common carbapenemases produced by CRE are KPC. Therefore, many of these new molecules such as ceftazidime-avibactam, meropenem-vaborbactam, imipenem-cilastatin-relebactam are preferred treatment options for non-urinary CRE infections.
According to 2022 IDSA guidelines, the best treatment options for patients with CRE infections receiving medical care in countries with a high prevalence of metallo-lactamase-producing bacteria
include ceftazidime-avibactam plus aztreonam, or cefiderocol as monotherapy.23
Ceftazidime-avibactam has in vitro activity against class A and D carbapenemases, but not against class B. IDSA recommendation for complicated infections which are sustained by non-class B CRE is based on several controlled trials showing non-inferiority of ceftazidime-avibactam compared to meropenem for UTI and IAI (RECLAIM trial), to doripenem for UTI (RECAPTURE trial) and to meropenem for nosocomial pneumonia (REPROVE trial).29,30,60
Nevertheless, none of these trials are specific for CRE infection whose involvement is extremely variable ranging from 13% to 28%. The type of carbapenemases is not indicated as well.
Since ceftazidime-avibactam has not activity against class B carbapenemases, there is recent evidence that combination of avibactam with aztreonam is active against these isolates.61 That synergic combination is particularly interesting because while aztreonam evades class B carbapenemases activity, avibactam inhibits other enzymes which are often co-expressed, conferring a pan-anti-carbapenemases activity.62 A phase III trial testing aztreonam-avibactam is currently underway for class B CRE,63 while some evidence exists suggesting ceftazidime-avibactam aztreonam as a useful clinical option.64 Alongside, some KPC mutations have been detected which can confer resistance to ceftazidime-avibactam. These mutations such as KPC-3 and KPC-2 are expressed mainly by K. Pneumoniae but also by E. Coli and should be taken into account.65
Anyway, ceftazidime-avibactam remains a useful option for treating serious KPC infections, even when used alone. This evidence is supported by many observational studies, even though further studies are needed to explore its efficacy in nosocomial pneumonias and any potential survival benefits of prolonging its infusion to more than 3 h.66
Meropenem-vaborbactam is a BLBLI active against KPC carbapenemase, but not against class B and D.
It was approved after TANGO I trial, which showed non-inferiority of meropenem-vaborbactam compared to piperacillin-tazobactam in patients with complicated UTI infections.67 Its limit was not selecting patients with CRE infections, since nearly all the pathogens analysed were sensible to meropenem. It was followed by TANGO II, which finally analysed CRE infections, including BSI, pyelonephritis, ventilator-associated pneumonia (VAP), and cIAI, showing a decrease in mortality and adverse event.68
A retrospective study by Ackley et al. shows no difference in clinical success when ceftazidime-avibactam or meropenem-vaborbactam are used in CRE infections.69 Moreover, some evidence suggests in-vitro synergy for the combination of meropenem-vaborbactam and aztreonam for the treatment of class B-producing CRE infections.63,70 Similarly, imipenem-relebactam is a recent drug combination approved by the FDA in 2019, which is only active against class A carbapenemases.
IDSA recommendations for mostly all CPE infection derive from RESTORE-IMI-1 and RESTORE-IMI2 trials.52,71 The first trial shows a better profile of imipenem-relebactam compared to imipenem plus colistin regarding clinical response rate, 28-day mortality rate and adverse events such as and treatment-associated nephrotoxicity. However, only 30% of pathogens involved were Enterobacteriaceae. The second trial shows non-inferiority of imipenem-relebactam in terms of 28-day mortality and clinical response, when compared with piperacillin-tazobactam, in patients with hospital acquired pneumonia (HAP) or VAP. Its in-vitro sinergy with aztreonam has been demonstrated too (63).
Cefiderocol is a novel siderophore cephalosporin enters the bacterial wall due to its high affinity for several penicillin-binding proteins, inhibiting cell wall formation. It represents an option for all Enterobacterales producing any of the five major carbapenemase enzymes.
Cefiderocol has a great safety profile, like other cephalosporins.72 European Medicines Agency (EMA) authorization includes MDR infections in patients with limited treatment options.73 However, CREDIBLE-CR trial, which compares cefiderocol with the best available treatment shows an increase in all-cause mortality in the cefiderocol group treated for BSI, pneumonia and sepsis.74
While these results seem to be due to the intrinsic mortality risk associated with Acinetobacter infections, the FDA has added a warning to its approval. At the same time, resistance to cefiderocol is starting to emerge and raising concern.75 In this case in vitro evaluation of CRE susceptibility is of crucial role for the choice of appropriate antibiotic therapy.
Plazomicin is a novel aminoglycoside which binds 30 s subunit of bacterial ribosomes and inhibits protein synthesis.76 Its spectrum of activity includes all carbapenemases but it is variably active against class B enzyme NDM-1.77 This novel aminoglycoside is approved by FDA since 2019, when the CARE trial demonstrated a reduction of mortality and fewer adverse events in patients with CRE-UTI treated with plazomicin, compared to colistin plus meropenem or tigecycline.78 Since the CARE trial was small, recommendations for BSI or pneumonia are not released. Plazomicin is not approved by EMA for financial reasons.
Eravacycline and omadacycline are tetracyclines with a broad spectrum of activity involving Gram-positive and Gram-negative bacteria except for Pseudomonas.61 In vitro analysis shows a greater sensibility when compared to tigecycline but they have more gastrointestinal adverse effect.79 Since phase III trials shows non-inferiority when compared to carbapenems, linezolid or moxifloxacin, eravacycline and omadacycline are approved for CPE-sustained IAI, SSI and pneumonia.80
As summarized, clinical options for the treatment of CPE infections are limited and all the antibiotics have a narrow spectrum of activity. The molecular pattern of each germ, which can be obtained rapidly, it’s not reliable enough to determine clinical susceptibility to each molecule and in vitro analysis has to be provided.
Since that, waiting for controlled trial, a definition of the best available approach is hard to provide. Morevoer, retrospective and observational study suggest controversial results about efficacy of monotherapy, and combination therapies have not been well defined yet. Generally, in severe infections when a CRE cause is suspected, empiric monotherapy seems to be insufficient and a combination therapy with the greatest coverage is judicious. Despite fast molecular definition, a rapid de-escalation should be performed only after in-vitro susceptibility analysis and monotherapy can be an option. However, the therapy sould be always discussed individually and tailored depending on microbiological environment, type of patients and source of infection.
ConclusionsSince the emergency of MDR bacteria, clinicians used increasingly powerful antibiotics with broader spectrum. The time necessary to conclude in vitro analysis of susceptibility leads to days of delay, during which unappropriated empiric therapy may facilitate resistance development. β-lactams and carbapenems have been continuingly used for their safety and activity but germs still manage to develop different resistance mechanisms which are nowadays hard to overcome.
ESBL-E and CRE are considered “supergerms” and are depicted as a microbiology priority. Finding a new application for non-β-lactam drugs is crucial to minimize further resistance development.
In this sense, the determination of molecular pattern of pathogens must be encouraged, as well as de-escalation.
Carbapenem-sparing strategy is a recent concept in treatment of infectious disease. The spread of CRE microorganisms has made this approach more urgent. The ultimate expansion of research towards novel molecules as BLBLI has enlarged therapeutic options but, depending their activity on molecular, lab and clinical details, their employ must be carefully examined. Similarly, novel tetracyclines and aminoglycosides are promising molecules because of their theoretic activity against a large group of ESBL and carbapenemases.
The complexity of susceptibility and germs resistance pattern is making infection control in the ICU increasingly challenging. The knowledge of the obstacles to overcome and of the limits and benefits of the available drugs will ensure antimicrobial monitoring and treatment for years to come.
Conflict of interestThe Authors of the study: “Treatment of severe infections caused by ESBL or carbapenemases-producing Enterobacteriaceae” (“Tratamiento de las infecciones severas causadas por Enterobacteriaceae productoras de beta lactamasas de espectro extendido (BLEE) y carbapenemasas.”) has no conflict of interest.