To evaluate the feasibility of using the Sentinella® portable gamma-camera for the diagnosis of brain death (BD).
DesignA prospective, observational feasibility study was carried out.
SettingIntensive Care Unit of a third level hospital.
PatientsConsecutive recording was made of the adults diagnosed with brain death based on clinical criteria following admission to the Intensive Care Unit in the period from January to December 2017.
InterventionsThe procedure was performed at the patient bedside with the intravenous administration of technetium 99 metastable hexamethylpropylene amine oxime. The absence of perfusion in the cerebral hemispheres and brainstem was described as a pattern consistent with BD. The diagnosis was correlated to the transcranial Doppler and/or electroencephalographic findings.
ResultsA total of 66.1% of the patients were men with an average age of 60 years [IQR: 51–72]. The most frequent causes resulting in BD were hemorrhagic stroke (48.2%, n=27), followed by traumatic brain injury (30.4%, n=17), ischemic stroke (10.7%, n=6) and post-cardiac arrest anoxic encephalopathy (7.1%, n=4). A clinical diagnosis of BD was made in all cases, and the portable gamma-camera confirmed the diagnosis in 100% of the patients with a pattern characterized by the absence of brain perfusion. In addition, the results were compared with the transcranial Doppler findings in 46 patients, confirming the presence of diastolic reverberation and/or systolic peaks. The electroencephalographic tracing was obtained in 10 cases, with the appearance of electrical silence, due to the absence of an acoustic window in the transcranial Doppler study.
ConclusionsA portable gamma-camera could be a useful and feasible tool for the diagnosis of BD.
Evaluar la factibilidad del empleo de la minigammacámara portátil Sentinella®, para el diagnóstico de muerte encefálica (ME).
DiseñoEstudio observacional, prospectivo, de factibilidad.
ÁmbitoUnidad de cuidados intensivos de un hospital de tercer nivel.
PacientesDesde enero a diciembre de 2017 se registraron de forma consecutiva los pacientes mayores de edad que tras su ingreso en unidad de cuidados intensivos fueron diagnosticados de ME según criterios clínicos.
IntervencionesEl procedimiento se realizó a la cabecera del paciente tras la administración intravenosa de tecnecio 99 metaestable-hexametil-propilen-amino-oxima. La ausencia de perfusión a nivel de hemisferios cerebrales y fosa posterior se describía como patrón compatible con ME. Se correlacionó el diagnóstico con doppler transcraneal y/o electroencefalograma.
ResultadosCincuenta y seis pacientes presentaron exploración física compatible con ME. Un 66,1% fueron hombres con una mediana de edad de 60 (RIQ: 51-72) años. La causa más frecuente que precipitó la ME fue el ictus hemorrágico en el 48,2% (27) seguido por traumatismo craneoencefálico grave en el 30,4% (17), ictus isquémico en el 10,7% (6) y encefalopatía anóxica tras parada cardiorrespiratoria en el 7,1% (4). En todos los casos se realizó el diagnóstico clínico de ME y posteriormente una gammagrafía portátil que confirmó dicho diagnóstico en el 100% de los pacientes. Se contrastó el resultado con doppler transcraneal en 46 de ellos que confirmaba la presencia de reverberación diastólica y/o picos sistólicos. En 10 casos se registró el electroencefalograma, con aparición de silencio eléctrico, debido a la ausencia de ventana acústica en el doppler transcraneal.
ConclusionesEl uso de minigammacámara portátil puede resultar una herramienta útil y factible para el diagnóstico de ME.
In clinical practice, the diagnosis of brain death (BD), understood as the irreversible cessation of all brain functions, is equivalent to death of the individual and thus requires responsibility and a concrete exploratory protocol to ensure diagnostic certainty with no margin of error.1–3 Rigor in methodical exploration is detailed in the clinical practice guides4 and is regulated in Spain by Royal Decree (RD) 1723 of 28 December 2012.5 The current regulations require two thorough clinical explorations spaced a variable period of time apart, or a single exploration accompanied by a complementary test,6 with the purpose of ensuring that the diagnostic process is as short as possible, avoiding organ deterioration, unexpected cardiac arrest and/or exhaustion of the family of the patient.7
The complementary tests are divided into those that evaluate brain function (electroencephalogram [EEG] and evoked potentials)8,9 and other techniques that measure brain blood flow,10–19 based on the determination of vascular circulation and/or tissue perfusion (arteriography, magnetic resonance angiography, computed tomography angiography, scintigraphy and transcranial Doppler ultrasound [TCD]).
From the physiopathological perspective, the absence on intracranial blood flow together with consistent clinical findings is representative of BD. In contrast, under certain circumstances that prevent the intracranial pressure from exceeding the systemic pressure (extensive craniotomy, ventricular shunting, major cranial trauma), some patients with BD may preserve a variable intracranial blood flow – though such flow is unable to maintain brain function and metabolism.20–22 Likewise, certain situations such as deep sedation, hypothermia and certain intoxications can result in a reversible absence of neuronal activity as determined by functional testing.23–25
The ideal characteristics required of a diagnostic test include26: objectivity and reliability, a high positive predictive value, great availability, low cost, low invasiveness and rapid performance in order to minimize the times. Other factors that are also important in the case of BD are the absence of relevant side effects in the patient or organs, and the absence of interference by central nervous system depressor drugs. Furthermore, the test must be applicable at the patient bedside, independent of the explorer, and should be easily reproducible. Table 1 summarizes the main advantages and limitations of each of the instrumental tests used in the diagnosis of BD.
Characteristics of the instrumental techniques used for the diagnosis of brain death.
| Instrumental test | EEG | EPs | Arteriography | TCD | CT-angiography | Scintigraphy |
|---|---|---|---|---|---|---|
| Invasive | No | No | Yes | No | Scantly invasive | Scantly invasive |
| Specificity | Low | Low | High | High | High | High |
| Sensitivity | High | High (depends on type of EP) | High | High | High | High |
| Patient transfer | No | No | Yes | No | Yes | Yes/No (portable) |
| Interpretation | Easy | Difficult | Easy | Easy | Easy | Easy |
| Other limitations | Artifacts with monitoring devicesDoes not detect subcortical structures | Artifacts with monitoring devicesNot useful in cases of prior deafness and/or petrosal bone fracture | False negatives in decompressive craniectomy and post-anoxic encephalopathyDoes not assess perfusionRequires vascular radiology room | Operator dependentAbsence of window in some patients (10–20%)False negatives in decompressive craniectomy and post-anoxic encephalopathy | False negatives in decompressive craniectomy and post-anoxic encephalopathy | Requires Department of Nuclear Medicine |
| Nee for contrast | No | No | Yes | No | Yes | No |
| Interference with sedatives | Yes | No | No | No | No | No |
TCD: transcranial Doppler ultrasound; EEG: electroencephalogram; EPs: evoked potentials.
Among the available diagnostic tests, brain perfusion gammagraphy using lipophilic tracers, such as metastable technetium 99 hexamethylpropylene amine oxime, have been extensively validated for the diagnosis of BD. Provided the procedure complies with the optimum quality standards, scintigraphy (gammagraphy or gamma scan) is a safe technique that is easy to perform and interpret, and affords a sensitivity and specificity of close to 100%.27 However, its use is currently limited, mainly because of the difficulty of moving the critically ill patient to the Department of Nuclear Medicine.
The present study evaluates the feasibility of using the Sentinella® portable gamma-camera (PGC) for the diagnosis of BD.
Material and methodsA prospective observational study was carried out in a 32-bed medical-surgical Intensive Care Unit (ICU) of a tertiary hospital serving as regional reference center for neurosurgery and interventional neurovascular procedures.
During a one-year period (January to December 2017), we consecutively registered those patients over 18 years of age diagnosed with BD according to clinical criteria, following their admission to the ICU. All subjects underwent systematic clinical evaluation and a complementary exploratory test (TCD or EEG, according to medical criterion). In addition, a scintigraphic study was made with the PGC in order to establish concordance between the findings.
Ethical aspectsThe study was carried out without interfering with or modifying patient clinical care. All patients or their representatives gave written consent for carrying out the procedure. The collection, filing and use of the personal information of the patients abided with current Spanish legislation (Organic Act 15/1999, of 13 December, referred to personal data protection). The study received no financial support, and was approved by the local Clinical Research Ethics Committee.
Technical aspectsElectroencephalography: The EEG tracing was recorded for 30min, following the technical recommendations of the American Clinical Neurophysiology Society.9 A 10-channel system (Neurofax, Nihon Kohden®) was used.
Transcranial Doppler ultrasound: A Multi-Dop® T Digital system was used for TCD, and in all cases the exploration was made by the same medical team. In all cases exploration was made through the temporal window, assessing both middle cerebral arteries (anterior circulation), and through the suboccipital window, assessing the vertebral and basilar arteries (posterior circulation). The ultrasound pattern corresponding to brain circulatory arrest was taken to be the presence of “inverted diastolic flow” and “systolic spikes”, following the recommendations of the Neurosonology Task Force28 and the Spanish Society of Neurosonology (Sociedad Española de Neurosonología)29 regarding the diagnosis of BD.
Brain perfusion scintigraphic evaluation protocol using the portable gamma-camera (PGC)Following the clinical diagnosis of BD, conduction of the test was requested from the Department of Nuclear Medicine, with a delay of about 30min (the time usually needed to prepare the radiodrug and move the PGC to our Unit). The study comprised 6 parts:
- 1.
Radiodrug production and quality control: 15min. Hexamethyl-propylen-amino-oxime was labeled with approximately 1110megabecquerels (MBq) of a solution for injection of 99mTc-sodium pernectate from a 99 molybdenum/metastable 99 technetium generator. Cobalt chloride was then added to stabilize the labeled compound. All labelings complied with the specifications for the eluate (pre-elution 24h and no more than 2h from elution). Quality control of the preparation consisted of visual inspection (compliant in all cases) and the determination of radiochemical purity after labeling using dual-run radiochromatography, with results of >90% lipophilic fraction in all the preparations. Use was made of a fixed dose of 925MBq±10% for adults, prepared immediately before administration.
- 2.
Transport of the radiodrug: Following preparation, transport and movement were kept to the essential minimum, with due justification in all cases. In this regard, we used shielded syringes and containers avoiding dispersion of the radioactive material into the environment, and which were easy to decontaminate. Use was made of transport circuits that as far as possible avoided coinciding with staff not related to the process.
- 3.
Administration of the radiotracer: The tracer was injected as a bolus dose through a central venous line using a shielded syringe, and followed immediately by the injection of 10ml of physiological saline solution.
- 4.
Imaging: Static images of the brain were acquired at 5min post-injection in both lateral projections, using the Sentinella® PGC. The acquisition time of each image was 300s; a Pinhole collimator measuring 2.5mm in diameter was used for this purpose. The sensitivity of the system with this collimator is 110cpm/μCi (counts per minute/microcuries) at a distance of 5cm, and 38cpm/μCi at a distance of 10cm – the spatial resolution at 3, 5 and 10cm being 5.4, 7.3 and 12.3mm, respectively.
- 5.
Analysis of the images: The images were interpreted by specialists in Nuclear Medicine. The absence of perfusion in the cerebral hemispheres and posterior fossa (“hollow skull” sign) was regarded as the scintigraphic pattern consistent with BD. It should be noted that throughout the study the patients maintained a systolic blood pressure of over 100mmHg, as confirmed by arterial monitoring.
- 6.
Post-scintigraphy care: Patient care was not modified by the ICU staff, though direct contact (a distance of under 30cm) during periods of over 10min was avoided as far as possible in the first hour after the exploration. This recommendation was agreed with the radioprotection service of the hospital, which conducted an area dosimetry analysis of the radiation received by the exposed professionals (Table 2). The dose received by a professional remaining 5min after injection of the radiotracer at a distance of less than 30cm from the patient during 10min would be 9.9μSv, which is equivalent to 1% of the annual population exposure limiting dose (1mSv/year) or 0.0495% of the annual occupational exposure limiting dose (20mSv/year).30
Table 2.Radiation received in each exploration by the exposed professionals according to distance from the patient, contact time and time after radiotracer injection.
Distance to patient after the procedure Contact(<30cm) 1m 2m 5m Dose rate (μSv/h) 60 3 0.75 0.12 Time after injection (min) 5 5 30 30 Time in contact with the patient (min) 10 30 60 180 Dose received per patient (μSv) 9.9 1.5 0.71 0.34 Public annual limit (mSv) 1mSv/year No. patients/year to reach public annual limit 101 673 1413 2943 Mean natural radiation dose in Spain 6.5μSv/day mSv: milliSievert; μSv: microSievert; μSv/h: microSievert/hour.
The data were reported as the mean, median or proportion, with the corresponding standard deviation (SD) or interquartile range (IQR), as applicable. The Statistical Package for the Social Sciences (SPSS), version 22.0 (IBM, Armonk, NY, USA), was used throughout.
ResultsDuring the period of the study, a total of 56 patients were diagnosed with BD according to clinical criteria. In all the patients use was made of EEG or TCD (according to medical criterion), as well as scintigraphy with the Sentinella® device (Table 3). Following the confirmation of BD, consent was obtained for organ donation, with an acceptance rate of 92%.
Principal demographic characteristics and test performed for the diagnosis of brain death.
| Age (median [IQR: years]) | 60 [IQR: 51–72] years |
| Gender (males [%]/females [%]) | 66.1% (37/56)/33.9% (19/56) |
| Time from admission to BD (median [IQR: h]) | 40 [IQR: 23.84]h |
| Arterial hypertension (%) | 57.1% (32/56) |
| Diabetes mellitus (%) | 30.4% (17/56) |
| Dyslipidemia (%) | 28.6% (16/56) |
| Transcranial Doppler (%) | 82.1% (46/56) |
| Electroencephalogram (%) | 17.8% (10/56) |
| Portable gamma-camera (%) | 100% (56/56) |
BD: brain death; IQR: interquartile range.
The median age of the patients diagnosed with BD was 60 years (IQR: 51–72), and 66.1% were males. The most common cause of death was vascular disease. Specifically, 17 patients were admitted due to severe traumatic brain injury (30.4%), four due to post-anoxic encephalopathy following cardiac arrest (7.1%), and 35 due to cerebrovascular disease (ischemic and/or hemorrhagic) (60.8%). The latter in turn included two patients with subarachnoid hemorrhage, 6 with extensive ischemic stroke, and 27 with hemorrhagic stroke (Table 4). The median time from admission to the diagnosis of BD was 40h 40 (IQR: 23–84).
We performed TCD in 46 patients (82.1%) – the observed flow pattern being consistent with BD. In the remaining 10 patients adequate ultrasound exploration of both the anterior and the posterior circulation in both hemispheres was not possible. We thus resorted to EEG as complementary diagnostic technique. In all cases the EEG tracing indicated the absence of brain bioelectrical activity.
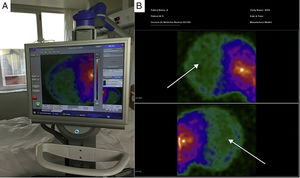
In the 56 patients studied, scintigraphic exploration with the PGC was carried out on site at the patient bedside (Fig. 1A). In all cases we confirmed the absence of perfusion of the cerebral hemispheres and posterior fossa (“hollow skull” sign) – this being consistent with clinically suspected BD (Fig. 1B). In only one patient did we have to resort to anteroposterior projection and correlation with previous computed tomography imaging due to doubtful and minimal activity in the peripheral right temporal region, corresponding to an area of trauma related to brain hematoma; the diagnosis of BD was thus finally confirmed. The procedure was well tolerated, and no complications were recorded.
Image A: portable gamma-camera (Sentinella®) exploration at the patient bedside. Image B: images in lateral projection obtained with the Sentinella® system. The arrows show the absence of radiotracer uptake in both the cerebral cortex and in the posterior fossa (“hollow skull” sign) – this finding being scintigraphically consistent with brain death.
The tests that help in diagnosing BD are generally used in patients that die as a result of severe brain injury, connected to a ventilator and to a range of different monitoring systems. Establishing a correct and early diagnosis is very important from the clinical and medical-legal perspective, and is of great relevance for possible organ donation.
Cerebral arteriography historically has been considered the gold standard for the diagnosis of BD. However, the procedure is complex, requires transfer of the patient to the neuroradiology room, may result in false-negative results in cases of decompressive craniotomy or in patients with ventricular drains, and implies the administration of contrast media with potential toxic effects upon organs that may be harvested for transplantation purposes. As a result, the technique has gradually become abandoned.13 On the other hand, the indication of computed tomography angiography has increased as a result of its widespread availability, but it has the same limitations as arteriography, since the patient must be moved outside the ICU, contrast administration is needed, and it is not possible to avoid false-negative results in situations of anoxic encephalopathy or decompressive craniotomy22 – with no advantages with respect to diagnosis using PGC.
In Spain, according to data from a multicenter descriptive study involving 1844 patients diagnosed with BD in 42 ICUs, the most commonly used complementary exploratory technique was EEG, followed by TCD. The latter technique has become the noninvasive exploratory option that has gained most popularity in recent years, and it is currently the first choice for assessing blood flow, despite its known limitations.31
The reviews published in recent years by groups of experts agree in pointing to brain perfusion scintigraphy as a first line option and the gold standard in this field. Indeed, in the United States it is the technique most commonly used to diagnose BD.32 One of its main advantages is that its sensitivity and specificity are close to 100%.33 Furthermore, it does not interfere with sedating drugs, is scantly invasive, and is able to assess both cerebral flow and perfusion, making interpretation of the images simple.34
However, although the usefulness of the technique has been widely demonstrated in the literature, its use is not so widespread in Spain. One of the main reasons for this is the need to move the patient to the Department of Nuclear Medicine, which is neither practical nor safe, and the fact that not all centers have a gamma-camera. However, in recent years there have been improvements in portable systems that allow scintigraphic exploration at the patient bedside,35 avoiding the need for transfer outside the ICU. Thus, the utilization of PGC has grown, and the technique is of demonstrated usefulness in sentinel node detection in breast cancer surgery, head and neck malignancies, and urological cancer.36 In the diagnosis of BD, the experience gained with PGC is still limited,37 but the technique could prove to be practical and reliable as an instrumental procedure for the diagnosis of BD, considering the results reported in the present study.
The PGC offers reliable information about the status of brain perfusion in terms of neuronal viability.38 Furthermore, it is important to underscore that performing and interpreting the exploration is simple, with results that are not influenced by those factors that commonly limit, complicate or preclude a clinical diagnosis, and the images obtained are recorded in a graphic document easily interpretable even by persons without specific prior training – provided the procedure complies with the required optimum quality standards.39
At present there are 34 systems similar to our own in Spain – this representing an important proportion of the Departments of Nuclear Medicine in the country. However, their indication for the diagnosis of BD is still limited. In 2009, Calvo et al.37 published the case of a patient diagnosed with BD using PGC, and described it as a useful diagnostic method, paving the way for larger studies seeking to determine the sensitivity and specificity of the technique. In our study, PGC showed excellent concordance with the other diagnostic procedures used (clinical exploration, EEG and TCD), with no discrepancies among the different methods, and obtaining a sensitivity and specificity of 100%.
On the other hand, the size of the PGC is similar to that of an ultrasound system, which in principle would allow it to be moved from one place to another. It could be interesting to develop logistics between centers to allow transfer of the PGC and/or the staff needed to establish the diagnosis at a distance from the reference centers.
One of the limitations facing implementation of the protocol could be the need for a radiopharmacy service for preparing the radiodrug. However, in relation to other indications of the technique, many Spanish Autonomous Communities have externalized preparation of the radioisotopes to companies able to supply them in a short period of time. Furthermore, most hospitals with a Department of Nuclear Medicine also have access to the required radioisotope, and the exploration as such requires no other specialized or sophisticated detection systems. On the other hand, a specialist in nuclear medicine must be available, though most such professionals are not physicians on duty. Nevertheless, most diagnoses of BD are made in the morning or afternoon shifts, making it possible for the staff to cover the requirements referred to this technique in their normal working hours. In this regard, it should be noted that 92% of the PGC studies made in our Department were performed before 8:00p.m., with only two explorations being requested outside this time period.
In view of the above, we believe that PGC may be an interesting alternative. Considering the relative ease of interpretation of the results, that fact that the technique is not operator dependent, is performed at the patient bedside, is not affected by sedating drugs, and poses no toxic effects for potentially transplantable organs, PGC could be regarded as a useful, feasible and safe tool for the diagnosis of BD.
Financial supportThe present study has received no financial support.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Moya Sánchez J, Royo-Villanova Reparaz M, Andreu Ruiz A, Ros Argente del Castillo T, Sánchez Cámara S, de Gea García JH, et al. Minigammacámara portátil para el diagnóstico de muerte encefálica. Med Intensiva. 2020;44:1–8.