To evaluate the use and effectiveness of a routine invasive strategy (RIS) in patients with acute coronary syndrome without persistent ST-segment elevation with renal dysfunction in the real world scenario.
MethodsA retrospective cohort study based on the ARIAM-SEMICYUC Registry (2011–2014) was carried out. Renal dysfunction was defined as GFR (Cockroft–Gault) <60ml/min (moderate dysfunction) or <30ml/min (severe dysfunction). Patients in which early angiography (<72h) was performed due to cardiogenic shock or recurrent myocardial ischemia were excluded. The primary endpoint was hospital mortality. Confounding factors were controlled using propensity score analysis.
ResultsA total of 4279 patients were analyzed, of which 26% had moderate renal dysfunction and 5% severe dysfunction. Patients with renal dysfunction had greater severity and comorbidity, higher hospital mortality (8.6 vs. 1.8%), and lesser use of the RIS (40 vs. 52%). The adjusted OR for mortality in patients without/with renal dysfunction were 0.38 (95% confidence interval [95%CI] 0.17–0.81) and 0.52 (95%CI 0.32–0.87), respectively (interaction p-value=.4779). The impact (adjusted risk difference) of RIS was higher in the group with renal dysfunction (−5.1%, 95%CI −8.1 to −2.1 vs. −1.6%, 95%CI −2.6 to −0.6; interaction p-value=.0335). No significant interaction was detected for the other endpoints considered (ICU mortality, 30-day mortality, myocardial infarction, acute renal failure or moderate/severe bleeding).
ConclusionsThe results suggest that the effectiveness of IRS is similar in patients with normal or abnormal renal function, and alert to the under-utilization of this strategy in such patients.
Evaluar la utilización y efectividad de la estrategia invasiva de rutina (EIR) en pacientes con síndrome coronario agudo sin elevación de ST con disfunción renal en el mundo real.
MétodosEstudio de cohortes retrospectivo basado en el registro ARIAM-SEMICYUC (años 2011–2014). Se consideró que había disfunción renal cuando el GFR (Cockroft-Gault) era menor de 60ml/min (disfunción moderada) o de 30ml/min (disfunción grave). Se excluyeron los pacientes en los que la coronariografía precoz (<72h) se debió a shock cardiogénico o isquemia recurrente. El desenlace primario fue la mortalidad hospitalaria. El control del confounding se realizó mediante un análisis de propensión.
ResultadosSe analizan 4.279 pacientes, de los cuales un 26% tenía disfunción renal moderada y un 5% disfunción grave. Los pacientes con disfunción renal presentaron una mayor gravedad y comorbilidad, una mayor mortalidad hospitalaria (8,6 frente a 1,8%) y una menor utilización de la EIR (40 frente a 52%). Las OR ajustadas mediante emparejamiento para pacientes sin/con disfunción renal fueron de 0,38 (intervalo de confianza al 95% [IC95%] de 0,17 a 0,81) y 0,52 (IC95% de 0,32 a 0,87), respectivamente (p de interacción 0,4779). El impacto de la EIR (diferencia de riesgos ajustada) fue mayor en el grupo con disfunción renal (−5,1%, IC95% entre −8,1 y −2,1, frente a −1,6%, IC95% entre −2,6 y −0,6, p de interacción=0,0335). Tampoco se detectó interacción significativa respecto a los demás enlaces considerados (mortalidad en UCI o a los 30 días, riesgo combinado de muerte o infarto, fracaso renal agudo o hemorragias moderadas/graves).
ConclusionesLos resultados evidencian que la efectividad de la EIR es similar en pacientes con función renal normal o reducida y alertan sobre una infrautilización de esta estrategia en estos últimos.
Patients presenting non-ST-segment elevation acute coronary syndrome (NSTE-ACS) with chronic renal dysfunction constitute a subgroup of individuals with a high mortality rate and an important risk of cardiac adverse events.1–8 Although the main clinical guides advocate the use of invasive management strategies in moderate-high risk patients9,10 the scientific literature consistently reflects a lesser utilization of invasive techniques in patients with NSTE-ACS and renal dysfunction.11–14
This discrepancy between the recommendations of the guides and routine clinical practice could be related to the uncertainty regarding the benefit-risk ratio of an early invasive strategy in patients with chronic renal failure. In effect, a metaanalysis of randomized trials on routine invasive strategies (RIS) versus conservative management in patients with a glomerular filtration rate (GFR) estimated using the Cockroft–Gault method of <60ml/min was only able to demonstrate a nonsignificant tendency toward lesser mortality with RIS.15 The problem of imprecision was greater in the subgroup of patients with severe renal dysfunction. In addition to these limitations, mention must be made of the risk of bias of the individual studies (characterized by a high cross-over rate) and the period in which they were carried out. In effect, a large proportion of the publications correspond to a period when many of the current antithrombotic agents and interventional technologies were not yet available; as a result, these results could be regarded as representing “indirect evidence”.16 On the other hand, it is well known that observational studies1,3,6,8,13,17,18 are hampered by confounding by indication. Although some studies address this problem through propensity score matching, in many cases the methodology used is not fully clear.
The present study uses data from a hospital acute coronary syndrome (ACS) registry to evaluate the frequency of use and the impact of RIS versus an initial conservative strategy in patients with NSTE-ACS, according to the degree of renal dysfunction.
MethodsPatientsThe study was based on data from the ARIAM-SEMICYUC registry,19 a voluntary registry of consecutive patients admitted to Spanish Coronary Units or Intensive Care Units (ICUs) with a diagnosis of ACS and over 18 years of age, with less than 48h from symptoms onset, and followed-up on until hospital discharge and (optionally) until 30 days from symptoms onset. Data compilation in the registry is carried out via annual cross-sectional sampling covering a period of three months. In addition, those centers that so wish can enter their own data over the full length of the year (continuous registry). A total of 76 centers participated during the study period; most corresponded to medium-size hospitals (Annex available in the online version), with the inclusion of an average of 49 patients per center. The study complied with Spanish legislation on data protection and the conduction of observational studies. The Ethics Committee did not consider it necessary to obtain patient informed consent.
In the present study we analyzed the patients included in the ARIAM-SEMICYUC registry between January 2011 and September 2014 with a diagnosis of NSTE-ACS. Patients admitted with cardiogenic shock (in which an urgent invasive strategy is recommended9) were excluded, as were those patients subjected to urgent coronarography due to shock or recurrent pain in the initial 72h following first contact with medical care.
MeasurementsThe patients were considered exposed if coronariography had been performed within the first 72h, and as non-exposed if otherwise. According to the established cutoff points, the patients were subclassified into three renal function groups: normal function (glomerular filtration rate [GFR] estimated with the Cockroft–Gault method20 ≥60ml/min), moderate dysfunction (GFR 30–59ml/min) or severe dysfunction (GFR<30ml/min).
The primary endpoint was in-hospital mortality. Secondary endpoints were mortality in the ICU and after 30 days, the appearance of infarction or reinfarction, moderate-severe hemorrhage (based on criteria of the GUSTO study21), and the appearance of acute renal failure (defined as a rise in creatinine to 1.5 times the baseline value, or diuresis <0.5ml/kg during 6h).
The following potential confounding factors were considered: demographic data (age, gender), care parameters (form of access, transport and delays in access to the center, availability of cardiac catheterization22), coronary risk factors, previous cardiovascular disease, previous treatments (pharmacological and coronary) and baseline clinical condition (blood pressure, heart rate, initial ECG tracing, initial Killip score,23 TIMI score,24 GRACE score25 and CRUSADE score26) (Tables 1 and 2).
Baseline characteristics of the patients.
| RIS (n=2075) | ICS (n=2204) | p-value | |
|---|---|---|---|
| Age in years, mean (SD) | 65.7 (12.6) | 68.3 (12.5) | <0.0001 |
| Gender (% females) | 539 (26.0) | 646 (29.3) | 0.0148 |
| Smoking | 718 (34.6) | 591 (26.8) | <0.0001 |
| Arterial hypertension | 532 (25.6) | 597 (27.1) | 0.2826 |
| Dyslipidemia | 1.135 (54.7) | 1.276 (57.9) | 0.0351 |
| Diabetes mellitus | 637 (30.7) | 778 (35.3) | 0.0014 |
| Previous myocardial infarction | 469 (22.6) | 618 (28.0) | <0.0001 |
| Arterial disease | 175 (8.4) | 233 (10.6) | 0.0173 |
| Previous heart failure | 79 (3.8) | 192 (8.7) | <0.0001 |
| COPD | 202 (9.7) | 257 (11.7) | 0.0419 |
| History of bleeding | 38 (1.8) | 77 (3.5) | 0.0008 |
| Known coronary lesions | 444 (21.4) | 570 (25.9) | 0.0006 |
| Previous aspirin use | 697 (33.6) | 867 (39.3) | <0.0001 |
| Previous use other antiplatelet drugs | 299 (14.4) | 379 (17.2) | 0.0126 |
| Previous use beta-blockers | 544 (26.2) | 704 (31.9) | <0.0001 |
| Previous use ACEIs | 868 (41.8) | 1004 (45.6) | 0.0142 |
| Previous use lipid-lowering drugs | 892 (43.0) | 1102 (50.0) | <0.0001 |
| Previous use diuretics | 365 (17.6) | 521 (22.6) | <0.0001 |
| Previous use nitrates | 210 (10.1) | 346 (15.7) | <0.0001 |
| Year | |||
| 2011 | 275 (13.3) | 482 (21.9) | <0.0001 |
| 2012 | 542 (26.1) | 573 (26) | |
| 2013 | 707 (34.1) | 654 (29.7) | |
| 2014 | 551 (26.6) | 495 (22.5) | |
| Availability of hemodynamics | 900 (43.4) | 514 (23.3) | <0.0001 |
| Private hospital | 99 (4.8) | 221 (10.0) | <0.0001 |
| First medical care contact | |||
| Hospital emergency room | 1011 (48.7) | 1019 (46.2) | 0.0015 |
| 112/061 | 255 (12.3) | 302 (13.7) | |
| Primary care center | 585 (28.2) | 575 (26.1) | |
| Hospitalized | 92 (4.4) | 154 (7.0) | |
| Others | 132 (6.4) | 154 (7.0) | |
| Delay in minutes, mean (SD) | 681.8 (518.9) | 627.5 (499.7) | <0.0001 |
| Glomerular filtration rate (Cockroft), mean (SD) | 85.6 (37.1) | 76.4 (35.7) | <0.0001 |
| Initial systolic blood pressure, mean (SD) | 140.0 (27.7) | 140.0 (26.3) | 0.7744 |
| Initial diastolic blood pressure, mean (SD) | 76.5 (16.5) | 77.1 (15.5) | 0.2113 |
| Initial heart rate, mean (SD) | 80.8 (21.5) | 77.6 (19.2) | <0.0001 |
| ECG upon admission | |||
| Normal | 235 (11.3) | 281 (12.8) | <0.0001 |
| ST deviation >0.5mm | 560 (27.0) | 544 (24.7) | |
| ST deviation <0.5mm | 251 (12.1) | 281 (12.8) | |
| Transient ST deviation | 193 (9.3) | 13 (5.9) | |
| T-wave inversion | 421 (20.3) | 433 (19.7) | |
| Trunk/multiple vessel pattern | 77 (3.7) | 42 (1.9) | |
| Others | 338 (16.3) | 492 (22.3) | |
| Initial Killip score | |||
| 1 | 1744 (84.1) | 1591 (72.2) | <0.0001 |
| 2 | 216 (10.4) | 355 (16.1) | |
| 3 | 115 (5.5) | 258 (11.7) | |
| TIMI score | |||
| 0–1 | 45 (2.2) | 41 (1.9) | <0.0001 |
| 1 | 479 (23.1) | 382 (17.3) | |
| 2 | 671 (32.3) | 742 (33.7) | |
| 3 | 461 (22.2) | 490 (22.2) | |
| 4 | 275 (13.3) | 356 (16.2) | |
| 5 | 122 (5.9) | 157 (7.1) | |
| 6–7 | 22 (1.1) | 36 (1.6) | |
| GRACE score, mean (SD) | 133.3 (36.6) | 142.2 (40.4) | <0.0001 |
| CRUSADE score, mean (SD) | 41.3 (17.9) | 37.9 (17.4) | <0.0001 |
SD: standard deviation; ECG: electrocardiogram; ICS: initial conservative strategy; RIS: routine invasive strategy; COPD: chronic obstructive pulmonary disease; ACEIs: angiotensin converting enzyme inhibitors.
Values reported as n (percentage), except where stated otherwise.
Standardized differences in the original cohorts and in the matched cohorts.
| Variable | GFR<60ml/min | GFR≥60ml/min | ||
|---|---|---|---|---|
| Original cohorts | Matched cohorts | Original cohorts | Matched cohorts | |
| Age | 2.139 | 1.274 | 17.638 | 12.827 |
| Gender | 8.126 | 5.786 | 5.226 | 7.504 |
| Arterial hypertension | 4.191 | 1.643 | 6.772 | 4.171 |
| Dyslipidemia | 9.472 | 2.46 | 4.201 | 2.628 |
| Diabetes | 7.478 | 0.811 | 5.563 | 2.15 |
| Previous infarction | 5.424 | 0 | 10.867 | 4.04 |
| Arterial disease | 8.186 | 0.586 | 3.237 | 2.808 |
| Heart failure | 24.099 | 5.715 | 13.52 | 6.18 |
| COPD | 9.862 | 2.308 | 3.918 | 5.979 |
| History of bleeding | 10.13 | 5.173 | 7.095 | 2.079 |
| Known coronary lesions | 5.34 | 0.872 | 9.443 | 3.069 |
| Previous aspirin use | 8.732 | 1.216 | 8.556 | 2.244 |
| Previous use other antiplatelet drugs | 0.935 | 2.838 | 5.231 | 1.605 |
| Previous use nitrates | 11.359 | 5.086 | 16.322 | 7.572 |
| Previous use beta-blockers | 8.463 | 5.003 | 11.572 | 6.059 |
| Previous use ACEIs | 0.159 | 1.612 | 6.341 | 4.339 |
| Previous use lipid-lowering drugs | 13.194 | 7.267 | 9.909 | 4.636 |
| Previous use diuretics | 9.039 | 4.248 | 11.529 | 5.668 |
| Year | 3.989 | 4.359 | 17.928 | 16.368 |
| Availability of hemodynamics | 43.997 | 26.012 | 38.535 | 46.102 |
| Private hospital | 26.862 | 0 | 7.116 | 8.313 |
| First contact | 7.724 | 3.395 | 5.893 | 7.54 |
| Delay | 9.565 | 5.323 | 10.715 | 9.646 |
| Glomerular filtration | 13.758 | 4.142 | 13.684 | 10.497 |
| Systolic blood pressure | 4.662 | 3.733 | 4.284 | 5.174 |
| Diastolic blood pressure | 2.913 | 3.732 | 1.769 | 0.721 |
| Heart rate | 14.498 | 3.646 | 12.124 | 7.873 |
| Normal initial ECG | 6.195 | 4.736 | 2.445 | 0.262 |
| ST deviation >0.5mm | 0.613 | 2.179 | 10.508 | 11.492 |
| ST deviation <0.5mm | 3.6 | 3.463 | 6.308 | 4.423 |
| Transient ST deviation | 6.45 | 2.37 | 14.365 | 15.87 |
| T-wave inversion | 4.331 | 7.197 | 1.972 | 5.014 |
| Trunk/multiple vessel pattern | 11.192 | 7.244 | 9.818 | 11.682 |
| Killip score | 31.888 | 12.878 | 15.435 | 6.466 |
| TIMI score | 5.677 | 1.357 | 10.07 | 3.407 |
| GRACE score | 20.393 | 8.443 | 10.825 | 2.992 |
| CRUSADE score | 26.452 | 9.784 | 6.788 | 0.435 |
ECG: electrocardiogram; COPD: chronic obstructive pulmonary disease; GFR: glomerular filtration rate; ACEIs: angiotensin converting enzyme inhibitors.
The effectiveness of RIS was examined by estimating the odds ratio (OR)–relative effect–and risk difference (RD)–absolute effect–with the corresponding 95% confidence intervals (95%CIs).
The control of confounding by indication was carried out via propensity score matching27,28 using the R29 and StatsDirect statistical packages.30 To this effect, we created a propensity score at the time of RIS that included 33 variables available at the time of patient admission (see Table S4 of the supplementary material available in the online version). The odds ratio of the RIS referred to the different outcomes or endpoints was analyzed through 5 quintile-stratified analysis of the propensity score and paired cohorts analysis. The absolute effect (RD) was estimated from the results of the 5 quintile-stratified propensity analysis, weighted by the method of Greenland and Robins.31
This analytical procedure was carried out separately for the patients with moderate-severe renal dysfunction (GFR<60ml/min) and for those with normal renal function (GFR≥60ml/min). The differences of effect (raw and adjusted) between these two strata were contrasted using an interaction test based on the Cochran Q-statistic.
In addition, a post hoc subgroups analysis was made referred to the GRACE score (>140 versus ≤140), gender (females versus males), and availability of hemodynamics (possibility or not of cardiac catheterization in the center).
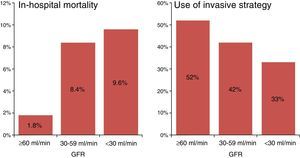
ResultsA total of 4363 patients were considered for inclusion in the study. Of these, 84 were excluded due to recurrent ischemia or cardiogenic shock. Of the 4279 patients finally analyzed, 69% had normal or slightly reduced renal function, 26% presented moderate renal dysfunction, and 5% had severe renal dysfunction. The observed in-hospital mortality rate was 1.8%, 8.4% and 9.6%, respectively (p<0.0001) (Fig. 1).
In-hospital mortality could not be determined in 287 patients with normal renal function (9.8%) and in 130 patients with renal dysfunction (9.7%). The demographic characteristics, comorbidities and initial severity of the patients lost to follow-up did not differ appreciably from those of the patients that completed follow-up (see Table S3 of the supplementary material available in the online version).
The percentage of patients subjected to RIS in the subgroups of normal renal function, moderate renal dysfunction and severe renal dysfunction was 52%, 42% and 33%, respectively (p<0.0001) (Fig. 1).
The baseline characteristics of the patient treated on an invasive or conservative basis were very different (Table 1). The conservative management group was characterized by patients preferentially admitted to hospitals without possibilities for hemodynamic testing, individuals of older age, with poorer renal function, an increased prevalence of diabetes and known ischemic heart disease, a lesser prevalence of high risk ECG findings, and greater initial severity as established from the Killip, TIMI or GRACE scores.
Propensity score matching of the cohorts yielded 1144 matched pairs with normal renal function and 499 with renal dysfunction. The distribution of the propensity scores in the invasive and conservative management groups showed adequate overlap (see Figure S1 of the supplementary material available in the online version). Propensity score matching was largely able to homogenize both populations (see Table S2 of the supplementary material available in the online version), though standardized differences of over 10 persisted in relation to some variables of little prognostic impact in both the subgroup with renal dysfunction and in the subgroup with normal renal function.
Regarding the primary endpoint, the effect of RIS was similar in patients with moderate-severe dysfunction (adjusted OR 0.52; 95%CI 0.32–0.87) and normal renal function (adjusted OR 0.38; 95%CI 0.17–0.81) (interaction test p=0.4779). Likewise, no interaction was observed between RIS and the degree of renal function on stratifying into three groups (interaction test p=0.76) (see Figure S3 of the supplementary material available in the online version).
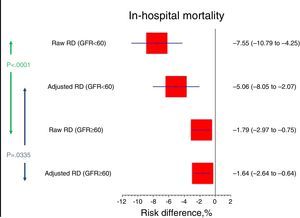
Given the different baseline risk, the risk difference was greater in the group of patients with renal dysfunction (RD −5.1%; 95%CI −8.1 to −2.1) than in the group with preserved renal function (RD −1.6%; 95%CI −2.6 to −0.6) (interaction test p=0.0335) (Tables 3 and 4; Fig. 2).
Effectiveness of the routine invasive strategy (raw and adjusted odds ratios).
| GFR<60 | GFR≥60 | Interaction p-value | |
|---|---|---|---|
| M ICU | |||
| ORr (95%CI) | 0.3734 (0.1781 to 0.7271) | 0.3973 (0.1758 to 0.8411) | 0.8996 |
| ORa (95%CI) | 0.44 (0.2165 to 0.8941) | 0.45 (0.2049 to 0.9882) | 0.9668 |
| ORas (95%CI) | 0.4224 (0.209 to 0.8537) | 0.444 (0.2048 to 0.9626) | 0.9255 |
| M Hosp | |||
| ORr (95%CI) | 0.4148 (0.26520 to 6352) | 0.3814 (0.1971 to 0.7071) | 0.8196 |
| ORa (95%CI) | 0.5227 (0.3157 to 0.8655) | 0.375 (0.1743 to 0.8066) | 0.4779 |
| ORas (95%CI) | 0.4579 (0.2736 to 0.7662) | 0.3392 (0.1679 to 0.6852) | 0.4998 |
| M30 | |||
| ORr (95%CI) | 0.4655 (0.2954 to 0.7204) | 0.3466 (0.1904 to 0.609) | 0.3999 |
| ORa (95%CI) | 0.549 (0.3462 to 0.8706) | 0.4412 (0.2403 to 0.8099) | 0.5743 |
| ORas (95%CI) | 0.5578 (0.3493 to 0.8907) | 0.3928 (0.218 to 0.7077) | 0.3608 |
| AMI reinf | |||
| ORr (95%CI) | 0.6359 (0.3104 to 1.2405) | 1.0084 (0.5927 to 1.7217) | 0.2634 |
| ORa (95%CI) | 0.52 (0.266 to 1.0164) | 1 (0.5807 to 1.7222) | 0.1375 |
| ORas (95%CI) | 0.7 (0.3649 to 1.343) | 1.1881 (0.7 to 2.0164) | 0.2166 |
| ARF | |||
| ORr (95%CI) | 0.7522 (0.5148 to 1.0893) | 0.6075 (0.2846 to 1.2584) | 0.5855 |
| ORa (95%CI) | 0.7213 (0.4895 to 1.0629) | 0.5789 (0.2755 to 1.2166) | 0.6069 |
| ORas (95%CI) | 0.8247 (0.5625 to 1.2092) | 0.7659 (0.3597 to 1.6307) | 0.8641 |
| Hemorr | |||
| ORr (95%CI) | 0.6447 (0.3285 to 1.2165) | 0.6407 (0.2756 to 1.45) | 0.9901 |
| ORa (95%CI) | 0.7143 (0.3682 to 1.3856) | 0.7857 (0.3567 to 1.7307) | 0.8563 |
| ORas (95%CI) | 0.844 (0.4366 to 1.6313) | 0.648 (0.2902 to 1.4473) | 0.6182 |
GFR: glomerular filtration rate; ARF: acute renal failure; Hemorr: moderate-severe hemorrhage; AMI reinf: infarction or reinfarction; 95%CI: 95% confidence interval; M Hosp: in-hospital mortality; M ICU: mortality in the Coronary Unit; M30: mortality after 30 days; ORr: raw odds ratio; ORa: matching adjusted odds ratio; ORas: stratification adjusted odds ratio.
Impact of the routine invasive strategy (raw and adjusted risk differences).
| GFR<60 | GFR≥60 | Interaction p-value | |
|---|---|---|---|
| M ICU | |||
| RDr (95%CI) | −4.81% (−7.26 to −2.34) | −0.95% (−1.38 to −0.13) | 0.0004 |
| RDa (95%CI) | −2.92% (−5.02 to −0.83) | −0.89% (−1.7 to −0.09) | 0.0763 |
| M Hosp | |||
| RDr (95%CI) | −7.55% (−10.79 to −4.25) | −1.79% (−2.97 to −0.75) | 0.0001 |
| RDa (95%CI) | −5.06% (−8.05 to −2.07) | −1.64% (−2.64 to −0.64) | 0.0335 |
| M30 | |||
| RDr (95%CI) | −0.0799 (−0.1211 to −0.0378) | −0.032 (−0.0494 to −0.0165) | 0.0364 |
| RDa (95%CI) | −0.0559 (−0.0974 to −0.0144) | −0.0262 (−0.0419 to −0.0105) | 1895 |
| AMI reinf | |||
| RDr (95%CI) | −0.0142 (−0.0335 to 0.0062) | 0.0002 (−0.0107 to 0.0108) | 0.9524 |
| RDa (95%CI) | −0.0123 (−0.0346 to 0.0101) | 0.0036 (−0.0074 to 0.0146) | 0.2109 |
| ARF | |||
| RDr (95%CI) | −0.0273 (−0.0603 to 0.0072) | −0.0058 (−0.0145 to 0.0021) | 0.2253 |
| RDa (95%CI) | −0.0185 (−0.0551 to 0.018) | −0.0028 (−0.0099 to 0.0044) | 0.4087 |
| Hemorr | |||
| RDr (95%CI) | −0.0165 (−0.0387 to 0.0066) | −0.0045 (−0.0132 to 0.0032) | 0.3289 |
| RDa (95%CI) | −0.006 (−0.0283 to 0.0163) | −0.0043 (−0.0121 to 0.0034) | 0.8878 |
GFR: glomerular filtration rate; RDa: adjusted risk differences; RDr: raw risk differences; ARF: acute renal failure; Hemorr: moderate-severe hemorrhage; AMI reinf: infarction or reinfarction; 95%CI: 95% confidence interval%; M Hosp: in-hospital mortality; M ICU: mortality in the Coronary Unit; M30: mortality after 30 days.
Regarding the secondary endpoints, RIS was associated to a decrease in mortality in the ICU and after 30 days, with no significant reduction in the risk of infarction/reinfarction, acute renal failure or moderate-severe hemorrhage. The effect for these 5 endpoints was similar (interaction test p=nonsignificant) for the patients with and without renal dysfunction (Table 3).
The analysis of subgroups showed that within the stratum of patients with a GRACE score >140, RIS reduced in-hospital mortality both in patients with renal dysfunction and in those with normal renal function. In the patients with a GRACE score ≤140, RIS did not significantly reduce in-hospital mortality. The interaction test p-value was not statistically significant, however. The effectiveness of RIS in patients with normal renal function was shown to be greater in centers without a Hemodynamics Unit than in those where hemodynamic testing was available (interaction test p=0.0133). There was no evidence of interaction between RIS application and patient gender in either patients with normal renal function or in patients with altered renal function (see Table S5 of the supplementary material available in the online version).
DiscussionOur results confirm the persistent scant use of RIS in patients with NSTE-ACS and renal dysfunction, and show that this strategy is associated to a relative reduction of in-hospital mortality and mortality after 30 days in these patients, being comparable to that of patients with normal renal function.5,6,8,15,32 In absolute terms, however, the short-term reduction in mortality among patients with renal failure is greater than in those with normal renal function: on treating the same number of patients, the number of deaths avoided by RIS is greater in patients with renal dysfunction than in individuals with normal renal function.
On the other hand, we detected no association between the application of RIS and the incidence of infarction, renal failure or moderate-severe hemorrhage in patients with renal dysfunction. The absence of a detectable effect upon the risk of infarction is consistent with the observations of most published studies, which report an initial increase in infarction risk that is only compensated by a decrease in reinfarction risk over the middle to long term. In turn, the negative results referred to the risk of hemorrhage and renal failure could be due to both a lack of statistical power of the study and to improved preventive practices in this field (e.g., generalization of the radial access, or the prevention of contrast-induced nephropathy).
With regard to the reasons underlying lesser utilization of RIS in patients with renal dysfunction, concerns about causing contrast-induced nephropathy and additional impairment of renal function could dissuade clinicians from applying RIS in such cases. However, the impact of iodine contrast administration upon important endpoints or outcomes (such as death or the need for dialysis) has not been clearly established,33 and the existing evidence points to a benefit-risk ratio favorable to the use of RIS in patients with renal failure.9,10
From a broader perspective, the infra utilization of RIS in these patients could be regarded as a peculiar manifestation of a tendency among clinicians to avoid using invasive procedures in high risk patients.11,12,14,17 In our concrete case, the utilization of RIS predominantly corresponded to the subgroup of patients with normal renal function and a GRACE score <140, which is precisely the group that stands to benefit least from such treatment. The possible influence of difficulties in moving the patient to the catheterization room, and the limitation of vital support measures in the more seriously ill patients, requires more in depth studies.
Our study has limitations, including particularly its observational design. Although propensity score matching resulted in a uniform distribution of the main covariables between the experimental group and the control group, we cannot rule out the existence of residual confounding effects attributable to certain variables not included in the registry. This residual confounding effect could explain the differences between the odds ratios estimated by observational studies and those estimated from randomized trials–with clearly more favorable values in the former. An alternative explanation could be the strong cross-over presence in the randomized studies (patients in the control group subjected to percutaneous coronary intervention), which would contribute to underestimate the benefit of RIS. In any case, rather than establishing a point estimation of the effect of RIS, our study sought to explore the possible heterogeneity of effects according to the degree of renal functional impairment.
Another limitation of our study refers to the losses to follow-up and the lack of middle-long term results. The credibility of our findings is strengthened by the absence of systematic differences between the patients lost to follow-up and those included in the study, as well as by the consistency of our results with those of other studies. Nevertheless, the possible existence of selection bias cannot be ruled out entirely.
Lastly, although we detected no subgroup effect in the patients with severe renal dysfunction, a total of 16 events were recorded among these subjects. Our study therefore has a problem of imprecision in estimating the effect in patients with severe renal dysfunction.34
In conclusion, the results obtained indicate that the effectiveness of RIS is similar in patients with normal or impaired renal function, and alert to infrautilization of this strategy among the latter type of patients.
Financial supportSEMICYUC.
Conflict of interestNone.
Please cite this article as: Latour-Pérez J, Gómez-Tello V, de-Miguel-Balsa E, Llamas-Álvarez A, Carrillo-López A, Sánchez-Román JA, et al. Estrategia invasiva de rutina en el síndrome coronario agudo sin elevación del segmento ST con disfunción renal. Resultados del registro ARIAM-SEMICYUC. Med Intensiva. 2016;40:280–288.